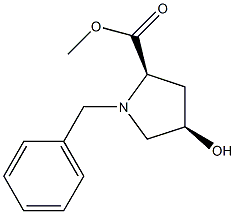
D-Proline, 4-hydroxy-1-(phenylmethyl)-, methyl ester, (4R)- synthesis
- Product Name:D-Proline, 4-hydroxy-1-(phenylmethyl)-, methyl ester, (4R)-
- CAS Number:114676-53-8
- Molecular formula:C13H17NO3
- Molecular Weight:235.279

100-39-0
429 suppliers
$10.00/10g
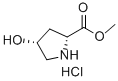
114676-59-4
111 suppliers
$6.00/250mg

114676-53-8
3 suppliers
inquiry
Yield:114676-53-8 233.4 g
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in chloroform at 0 - 75; for 1 h;
Steps:
B Step B. Methyl (2R,4R)-1-benzyl-4-hydroxpyrrolidine-2-carboxylate
Methyl (2R,4R)-4-hydroxypyrrolidine-2-carboxylate hydrochloride (1.19 mol) was suspended in CHCl3 (2 L) in four-neck flask equipped with mechanical stirrer, reflux condenser, dropping funnel and septum and was cooled to 0° C. DIPEA (520 mL, 2.98 mol) was added dropwise at 0° C. and the reaction mixture was warmed to room temperature.
Benzyl bromide (141.7 mL, 1.19 mol) was added dropwise for 1 h.
The reaction mixture was heated to reflux (75° C., oil bath temp.) for 1 h and cooled to room temperature (water bath with crushed ice).
Water (1 L) was added to the reaction mixture and layers were separated.
Organic layer was washed with water (1*1 L), 1M NaOH (1*1 L), brine (1*1 L), dried over MgSO4, filtered and concentrated under reduced pressure to give 233.4 g (83%, after 2 steps) of the desired as a pale orange oil. ESI+MS m/z=235.95 (M+1)+; 1H NMR (700 MHz, CDCl3) δ 7.32-7.23 (m, 4H), 4.24 (m, 1H), 3.86 (d, J=13.1 Hz, 1H), 3.71 (d, J=13.1 Hz, 1H), 3.63 (s, 1H), 3.34 (dd, J=10.1, 3.8 Hz, 1H), 3.11 (d, J=10.7 Hz, 1H), 3.01 (dt, J=9.9, 1.6 Hz, 1H), 2.63 (dd, J=10.1, 3.8 Hz, 1H), 2.37 (ddd, J=14.2, 10.0, 5.7 Hz, 1H), 1.95 (m, 1H).
References:
US2019/300525,2019,A1 Location in patent:Paragraph 0448; 0451; 0452

100-39-0
429 suppliers
$10.00/10g
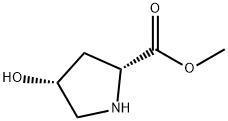
114676-47-0
50 suppliers
$95.00/250mg

114676-53-8
3 suppliers
inquiry

100-44-7
654 suppliers
$13.50/250G

114676-59-4
111 suppliers
$6.00/250mg

114676-53-8
3 suppliers
inquiry

2584-71-6
334 suppliers
$6.00/1g

114676-53-8
3 suppliers
inquiry
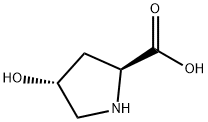
51-35-4
882 suppliers
$6.00/5g

114676-53-8
3 suppliers
inquiry