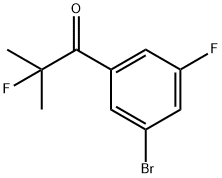
1-(3-Bromo-5-fluorophenyl)-2-fluoro-2-methylpropan-1-one synthesis
- Product Name:1-(3-Bromo-5-fluorophenyl)-2-fluoro-2-methylpropan-1-one
- CAS Number:1147871-75-7
- Molecular formula:C10H9BrF2O
- Molecular Weight:263.08
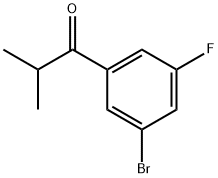
1147871-74-6
18 suppliers
inquiry

1147871-75-7
5 suppliers
$908.00/250mg
Yield:-
Reaction Conditions:
Stage #1: 1-(3-bromo-5-fluorophenyl)-2-methylpropan-1-onewith sodium pentanolate in N,N-dimethyl-formamide at 10 - 20; for 1.91667 h;
Stage #2: with tert-butyldimethylsilyl chloride in N,N-dimethyl-formamide at 10 - 28; for 1 h;
Stage #3: with water;Selectfluormore than 3 stages;
Steps:
2.B; 2
To a 100 L round bottomed flask fitted with a thermocouple, under an atmosphere of N2 was added sequentially sodium amylate (3.0 kg, 27.3 mol) and DMF (11 L). The slurry was aged for 30 minutes until almost all of the base had dissolved. Then the solution was cooled to 10 0C and a ~50 weight % solution of the isopropyl ketone 12 (10.8 kg, 50 weight %, 5.4 kg, 22 mol) was added over 55 minutes while maintaining the temperature between 15 - 20 0C via the controlled addition of the isopropyl ketone. Upon complete addition, the solution was aged for 30 minutes, cooled to 10 0C and treated with TBSCl (4.3 kg, 28.5 mol) over a 30 minute period while maintaining the temperature between 20 - 28 0C via controlled TBSCl addition. The reaction was aged for 30 minutes, then Select-Fluor (8.5 kg, 24 mol, Air Products) was then added over 1.5 hours while maintaining the reaction temperature 28 - 35 0C and the slurry aged for 1 hour. On complete conversion, water (18 L) was added, followed by toluene (12 L). The resulting biphasic mixture was transferred to a 100 L cylindrical vessel and aged for 10 minutes. The layers were separated and the organic phase was washed with additional water (9 L). The wet toluene solution containing fluoro ketone 13 was held for 36 hours while a second batch of equal size was processed. The combined toluene batches containing fluoro ketone 13 were then concentrated until the KF < 200 ppm and about 50 weight %. The resulting toluene solution of fluoro ketone 13 was used directly in the next step. HPLC retention time fluoro ketone 13 = 8.5 minutes on 25 cm Zorbax SB C-18, using MeCN / 0.1 H3PO4, 1 mL / min. gradient elution: 70% MeCN for 5 minutes, then to 90% MeCN at 10 minutes, hold for 5 minutes at 210 nm.IR vmax / cm"1 3083 (C-H), 2989 (C-H), 2941 (C-H), 1696 (C=O), 1577 (C=C); 1H NMR (500 MHz, CDCl3) δ 8.02 (IH, s, ArH), 7.74 (IH, br d, J^ = 9.1 Hz, ArH), 7.65 (IH, dt, 3HF = 7.66 Hz, JH.H = 2.0 Hz, ArH), 1.69 (6H, d, 3H.F = 21.7 Hz, 2 x CH3); 13C NMR (125 MHz, CDCl3) δ 198.2 (dd, J = 27.1 Hz, 1.8 Hz, C=O), 162.2 (d, J = 252.3 Hz, ArCF), 136.9 (dd, J = 6.8, 4.3 Hz, ArCCO), 128.9 (dd, J = 8.6, 3.1Hz, ArCH), 123.5 (d, J = 24.6 Hz, ArCH), 122.8 (dd, J = 9.2, 1.2 Hz, ArCBr), 115.8 (dd, J = 22.8, 9.2 Hz, ArCH), 99.9 (d, J = 180.3 Hz, C(CH3)2), 25.5 (d, J = 24.0 Hz, 2 x CH3); 19F NMR (470 MHz, CDCl3) δ -109.96, Ar-F), -144.68 (C-F).
References:
WO2009/54923,2009,A2 Location in patent:Page/Page column 34; 35; 36