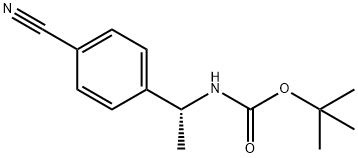
N-[(1R)-1-(4-Cyanophenyl)ethyl]carbamic acid tert-butyl este synthesis
- Product Name:N-[(1R)-1-(4-Cyanophenyl)ethyl]carbamic acid tert-butyl este
- CAS Number:1149727-73-0
- Molecular formula:C14H18N2O2
- Molecular Weight:246.3
![(R)-[1-(4-Bromophenyl)ethyl]carbamic acid tert-butyl ester](/CAS/GIF/578729-21-2.gif)
578729-21-2
88 suppliers
$30.00/100mg
557-21-1
99 suppliers
$12.00/5g
![N-[(1R)-1-(4-Cyanophenyl)ethyl]carbamic acid tert-butyl este](/CAS/GIF/1149727-73-0.gif)
1149727-73-0
9 suppliers
inquiry
Yield:-
Reaction Conditions:
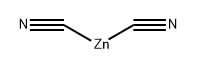
Stage #1:[(R)-1-(4-bromo-phenyl)-ethyl]-carbamic acid tert-butyl ester;dicyanozinc in N,N-dimethyl-formamide for 1 h;
Stage #2:tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 70 - 75; for 20.5 h;
Steps:
4
To a vial was charged tert-butyl [(1R)-1-(4-bromophenyl)ethyl]carbamate (4.3 gm), zinc cyanide (1.18 gm), and dimethylfomamide (13 mL). The mixture was purged with a stream of nitrogen then stirred under a nitrogen atmosphere for 1 hour. The reaction was then treated with palladium tetrakis(triphenylphosphine) (0.5 gm), sealed, and heated to 75 0C for 3.5 hours. The reaction was then heated at 70 0C for 17 hours. The mixture was cooled to room temperature and diluted with toluene (50 mL). Thiocyanuric acid (0.26 gm) was added followed by 3% aqueous sodium hydroxide (70 mL). The organic phase was washed again with a solution of thiocyanuric acid (0.26 gm) in 3% aqueous sodium hydroxide (70 mL). The organic phase was dried over sodium sulfate, filtered through a pad of Celite, concentrated under reduced pressure, and finally in vacuo to give a dark oil (3.9 gm). This material was diluted with dichloromethane and purified on a Biotage 65i column by elution with a gradient of 0-80% ethyl acetate/heptanes to give the title compound as a colorless oil (3.0 gm): 1H NMR (400 MHz, CDCI3) δ ppm 7.52 - 7.69 (2 H, m), 7.41 (2 H, m), 4.83 (2 H, m), 1.21 - 1.52 (12 H, m).
References:
PFIZER INC. WO2009/60278, 2009, A1 Location in patent:Page/Page column 48