
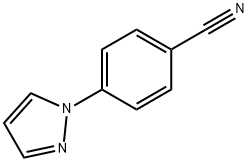
4-(5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)benzonitrile synthesis
- Product Name:4-(5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)benzonitrile
- CAS Number:1150620-53-3
- Molecular formula:C16H18BN3O2
- Molecular Weight:295.14
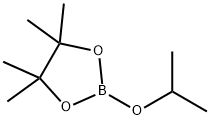
61676-62-8
321 suppliers
$11.19/5G
25699-83-6
116 suppliers
$7.00/250mg

1150620-53-3
14 suppliers
inquiry
Yield:1150620-53-3 86.28%
Reaction Conditions:
Stage #1: 4-pyrazol-1-yl-benzonitrilewith 2,2,6,6-tetramethyl-piperidine;n-butyllithium in tetrahydrofuran;hexane at -50 - 0;
Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane in tetrahydrofuran;hexane at -50 - 20;Product distribution / selectivity;
Steps:
5.g 5g: 4-[5-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-pyrazol-1-yl]-benzonitrile
5g: 4-[5-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-pyrazol-1-yl]-benzonitrile To 2,2,6,6-tetramethylpiperidine (24.07 g) was added tetrahydrofuran (115 mL). The reaction mixture was cooled to -20° C. At this temperature 1.6 M n-butyl lithium in hexanes (102 mL) was added, maintaining the temperature at less than 0° C. The reaction mixture was stirred at 0° C. for thirty minutes. The reaction mixture was cooled to -50° C. At this temperature a pre-mixed solution of 4-(1H-pyrazol-1-yl)benzonitrile (23 g) dissolved in tetrahydrofuran (115 mL) was added over approximately ten minutes, maintaining the temperature at less than -50° C. Tetrahydrofuran (12 mL) was added, and the reaction was stirred at -50° C. for at least thirty minutes. 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (35 mL) was charged over approximately twenty-five minutes, whilst maintaining the temperature at less than -50° C. The reaction mixture was stirred for approximately thirty minutes at -50° C., and then allowed to warm to approximately -15° C. Acetic acid (23.5 mL) was added over approximately twenty minutes, maintaining the temperature at less than 0° C. For convenience the reaction mixture was allowed to warm to approximately 20° C. over approximately sixteen hours. The reaction mixture was cooled to 0° C. and water (276 mL) was charged over approximately thirty-five minutes, maintaining the temperature at less than 0° C. The reaction mixture was stirred for an additional thirty minutes. The product was isolated by filtration and washed (on the filter) with water (46 mL) and then with n-heptane (46 mL). The damp solid was then dried in vacuo at 40° C. for approximately twenty-four hours to give the title product (34.62 g, 86.28%); 1H NMR (500 MHz, CDCl3% 1.23 (s, 12H), 6.90 (d, J=1.7 Hz, 1H), 7.69-7.63 (m, 4H), 7.70 (d, J=1.7 Hz, 1H). 13C NMR (125 MHz, CDCl3): 143.4, 140.4, 131.5, 123.8, 118.4, 117.5, 109.7, 83.7, 67.0, 23.6.
References:
US2011/224229,2011,A1 Location in patent:Page/Page column 19