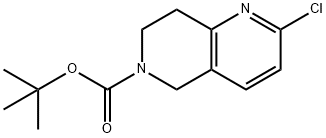
tert-butyl 2-chloro-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate synthesis
- Product Name:tert-butyl 2-chloro-7,8-dihydro-1,6-naphthyridine-6(5H)-carboxylate
- CAS Number:1151665-15-4
- Molecular formula:C13H17ClN2O2
- Molecular Weight:268.74
766545-20-4
135 suppliers
$50.00/1g

24424-99-5
858 suppliers
$13.50/25G

1151665-15-4
93 suppliers
$45.00/100mg
Yield: 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20; for 2 h;
Steps:
1
To a slurry of 2-chloro-5,6,7,8-tetrahydro-1,6-naphthyridine hydrochloride (106.1 g, 517 mmol, commercially available from D-L Chiral Chemicals, ST-0143) and N,N-diisopropylethylamine (80 g, 108 mL, 621 mmol, 1.2 eq) in DCM (1 L) was added a solution of di-tert-butyl dicarbonate (119 g, 543 mmol, 1.05 eq) in DCM (100 mL) via an addition funnel within 1 hr. The reaction mixture became a clean solution and the solution thus obtained was stirred at room temperature for an additional hour and monitored using LCMS. Upon completion, the reaction mixture was concentrated. The residue was dissolved in EtOAc (1 L) and washed with water (3*300 mL), washed with brine (300 mL) and dried over Mg504. The solvent was evaporated under vacuum to give the title compound as an off-white solid (139 g, yield: 100%). 1H NMR (400 MHz, CDCl3) δ ppm 1.49 (9H, s), 2.97 (2H, t, J=5.9 Hz), 3.73 (2H, t, J=6.0 Hz), 4.57 (2H, s), 7.17 (1H, d, J=8.0 Hz), 7.38 (1H, d, J=8.0 Hz) ppm; LCMS m/z: 269 (M+1).
References:
Amgen Inc. US2012/244110, 2012, A1 Location in patent:Page/Page column 61
210539-04-1
86 suppliers
$276.00/250mg

24424-99-5
858 suppliers
$13.50/25G

1151665-15-4
93 suppliers
$45.00/100mg