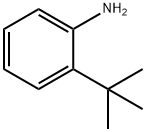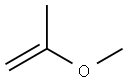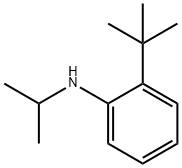
Benzenamine, 2-(1,1-dimethylethyl)-N-(1-methylethyl)- synthesis
- Product Name:Benzenamine, 2-(1,1-dimethylethyl)-N-(1-methylethyl)-
- CAS Number:1152959-44-8
- Molecular formula:C13H21N
- Molecular Weight:191.31
Yield:1152959-44-8 100%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in 1,2-dichloro-ethane at 60;Inert atmosphere;
Steps:
104.1
Step 1 : 2-(tert-butyl)-N-isopropylaniline:To a solution of 2-(tert-butyl)aniline (1 .045 ml, 6.70 mmol) in 1 ,2-Dichloroethane (10 ml) was added propan-2-one ( 0.541 ml, 7.37 mmol), followed by sodiumtriacetoxyborohydride ( 2.27 g, 10.72 mmol) and acetic acid ( 0.422 ml, 7.37 mmol). This mixture was heated to 60 °C overnight under a nitrogen atmosphere. After cooling to RT the reaction mixture was partitioned between DCM and water. The organic phase was dried (phase separator cartridge) and concentrated in vacuo to afford the desired product (1 .53 g, 100%) as an orange oil.1 H NMR (500 MHz, CDCI3): 7.26 (1 H, dd), 7.14 (1 H, m), 6.68-6.72 (2H, m), 3.76 (1 H, m), 1 .46 (9H, s), 1 .29 (6H, d).
References:
WO2012/69852,2012,A1 Location in patent:Page/Page column 135