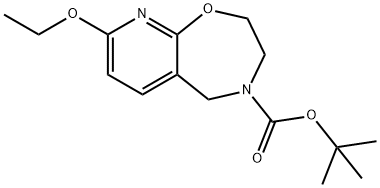
Pyrido[3,2-f]-1,4-oxazepine-4(5H)-carboxylic acid, 8-ethoxy-2,3-dihydro-, 1,1-diMethylethyl ester synthesis
- Product Name:Pyrido[3,2-f]-1,4-oxazepine-4(5H)-carboxylic acid, 8-ethoxy-2,3-dihydro-, 1,1-diMethylethyl ester
- CAS Number:1154471-53-0
- Molecular formula:C15H22N2O4
- Molecular Weight:294.35

64-17-5
706 suppliers
$10.00/50g
![tert-butyl 8-chloro-2,3-dihydropyrido[3,2-f][1,4]oxazepine-4(5H)-carboxylate](/CAS/GIF/956434-30-3.gif)
956434-30-3
37 suppliers
$21.00/100mg
![Pyrido[3,2-f]-1,4-oxazepine-4(5H)-carboxylic acid, 8-ethoxy-2,3-dihydro-, 1,1-diMethylethyl ester](/CAS/GIF/1154471-53-0.gif)
1154471-53-0
2 suppliers
inquiry
Yield:1154471-53-0 30%
Reaction Conditions:
Stage #1: ethanolwith sodium hydride in toluene at 70; for 0.25 h;Inert atmosphere;
Stage #2: tert-Butyl 8-chloro-2,3-dihydropyrido[3,2-f][1,4]oxazepine-4(5H)-carboxylate;tris-(dibenzylideneacetone)dipalladium(0);2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 100; for 2 h;Inert atmosphere;
Steps:
13.1
(step 1) To a solution of ethanol (0.10 mL) in toluene (4 mL) was added sodium hydride (0.14 g), and the resulting mixture was stirred at 70°C for 15 min under a nitrogen atmosphere. A mixture of tert-butyl 8-chloro-2,3-dihydropyrido[3,2-f][1,4]oxazepine-4(5H)-carboxylate (0.50 g), BINAP (0.033 g), Pd2(dba)3 (0.024 g) and toluene (4 mL) was added, and the resulting mixture was stirred at 100°C for 2 hr under an argon atmosphere. The reaction solution was poured into water, and the resulting product was extracted with ethyl acetate. The organic layer was washed with water and saturated brine and dried, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (solvent gradient; 0→40% ethyl acetate/hexane) to give tert-butyl 8-ethoxy-2,3-dihydropyrido[3,2-f][1,4]oxazepine-4(5H)-carboxylate (0.16 g, 30%) as a colorless oil. 1H-NMR(CDCl3):δ1.36(3H,t,J=7.2Hz), 1.42(9H,s), 3.80-3.83(2H,m), 4.32(2H,q,J=7.2Hz), 4.15-4.43(4H,m), 6.43(1H,d,J=8.1Hz), 7.40-7.52(1H,m)
References:
EP2213675,2010,A1 Location in patent:Page/Page column 36; 37