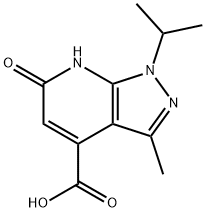
1-ISOPROPYL-3-METHYL-6-OXO-6,7-DIHYDRO-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID synthesis
- Product Name:1-ISOPROPYL-3-METHYL-6-OXO-6,7-DIHYDRO-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID
- CAS Number:1160246-29-6
- Molecular formula:C11H13N3O3
- Molecular Weight:235.24
![ETHYL 1-ISOPROPYL-3-METHYL-6-OXO-6,7-DIHYDRO-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLATE](/CAS/GIF/1174844-27-9.gif)
1174844-27-9
8 suppliers
inquiry
![1-ISOPROPYL-3-METHYL-6-OXO-6,7-DIHYDRO-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID](/CAS/GIF/1160246-29-6.gif)
1160246-29-6
15 suppliers
inquiry
Yield:1160246-29-6 51.38 g
Reaction Conditions:
with water;sodium hydroxide in tetrahydrofuran;ethanol at 40; for 0.666667 h;
Steps:
2.2
Step 2:To a solution of diethyl 2-oxobutanedione (176 g, 0.94 mol) in toluene (2 L) was added 3 -methyl- l-(l-methylethyl)-lH-pyrazol-5 -amine (82.5 g, 0.59 mol), and the mixture stirred at 62 °C, overnight. After cooling to room temperature, the mixture was concentrated in vacuo and the crude residue dissolved into acetic acid (1.5 L). The mixture was heated at reflux for 2 h. After cooling to room temperature, the mixture was concentrated in vacuo to afford a solid residue, which was recrystallized from DCM to afford the desired product as a yellow colored solid. The collected solid was suspended in ethanol (1510 niL) and THF (216 mL) followed by addition of 3N NaOH (334 mL) and the reaction mixture was stirred at 40 °C for 40 min. The mixture was concentrated in vacuo to remove the volatiles and the aqueous phase acidified using IN HC1. The resulting precipitate was collected by filtration and dried under high vacuum to give the title compound, 3-methyl-l-(l-methylethyl)-6-oxo-6,7-dihydro-lH-pyrazolo[3,4-?]pyridine-4- carboxylic acid, as 51.38 g. LCMS E-S (?+?) = 236.1. 1H NMR (400 MHz, DMSO-d6) ? ppm 1.35 (d, J=6.8 Hz, 6 H), 2.41 (s, 3H), 4.84-4.91 (m, 1H), 6.64 (s, 1H). Carboxylic acid proton not observed.
References:
WO2013/39988,2013,A1 Location in patent:Page/Page column 46-47
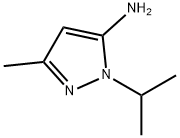
1124-16-9
89 suppliers
$35.00/200μmol
![1-ISOPROPYL-3-METHYL-6-OXO-6,7-DIHYDRO-1H-PYRAZOLO[3,4-B]PYRIDINE-4-CARBOXYLIC ACID](/CAS/GIF/1160246-29-6.gif)
1160246-29-6
15 suppliers
inquiry