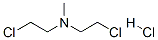6-Bromo-1'-methylspiro[indoline-3,4'-piperidin]-2-one synthesis
- Product Name:6-Bromo-1'-methylspiro[indoline-3,4'-piperidin]-2-one
- CAS Number:1160248-46-3
- Molecular formula:C13H15BrN2O
- Molecular Weight:295.17
Yield:1160248-46-3 82%
Reaction Conditions:
Stage #1: 6-bromo-2,3-dihydro-1H-indole-2-onewith sodium hexamethyldisilazane in tetrahydrofuran at -78; for 0.5 h;
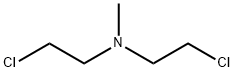
Stage #2: mechlorethamine hydrochloride in tetrahydrofuran at -78 - 20; for 48.5 h;
Steps:
4.1.3. Synthesis of 11
General procedure: To a solution of 5-bromo-1,3-dihydro-2H-indole-2-one (1.272 g, 6 mmol) in THF(15 ml) was added a solution of NaN(SiMe3)2 in THF (30 ml, 30 mmol). The reaction mixture was cooled to -78 °C and stirring was continued at the same temperature for 0.5 h. The 2-chloro-N-(2-chloroethyl)-N-methyl ethylamine hydrochloride (1.155 g, 6 mmol) was added to the mixture, continued to stir at -78 °C for 0.5 h, then stirred for 2 d at room temperature (TLC control). 4 M HCl (10 ml) was added, the mixture was adjusted pH to 10 with concentrated ammonia and extracted with CH2Cl2 (3 × 20 ml). The combined organic layer was dried (3 g of Na2SO4) and concentrated to afford the crude product, which was purified by FC with MeOH/CH2Cl2.
References:
Ye, Lianbao;Tian, Yuanxin;Li, Zhonghuang;Jin, Hong;Zhu, Zhengguang;Wan, Shanhe;Zhang, Junyan;Yu, Pengjiu;Zhang, Jiajie;Wu, Shuguang [European Journal of Medicinal Chemistry,2012,vol. 50,p. 370 - 375] Location in patent:experimental part
![tert-Butyl 6-broMo-2-oxospiro[indoline-3,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/1160247-29-9.gif)
1160247-29-9
41 suppliers
$65.00/10mg
![6-Bromo-1'-methylspiro[indoline-3,4'-piperidin]-2-one](/CAS/20210111/GIF/1160248-46-3.gif)
1160248-46-3
5 suppliers
inquiry