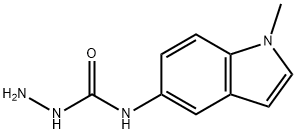
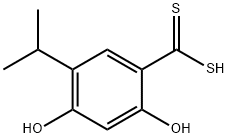
1-(2,4-dihydroxy-5-isopropylphenylcarbonothioyl)-4-(1-Methyl-1H-indol-5-yl)seMicarbazide synthesis
- Product Name:1-(2,4-dihydroxy-5-isopropylphenylcarbonothioyl)-4-(1-Methyl-1H-indol-5-yl)seMicarbazide
- CAS Number:1160618-89-2
- Molecular formula:C20H22N4O3S
- Molecular Weight:398.48
Yield:1160618-89-2 59%
Reaction Conditions:
Stage #1: 2,4-dihydroxy-5-isopropylbenzenecarbodithioic acidwith sodium hydrogencarbonate;chloroacetic acid in N,N-dimethyl-formamide at 0 - 20; for 1.05 h;
Stage #2: N-(1-methyl-1H-indol-5-yl)hydrazinecarboxamide in N,N-dimethyl-formamide at 20 - 80; for 1.05 h;Product distribution / selectivity;
Steps:
2
STEP-2: To a stirred suspension of 5Og (220mmols) of 2 and 55.5g (660mmols) of NaHCO3 in 65OmL of anhydrous DMF was added 21g (220mmols) of chloroacetic acid in 3 portions (for 3mins.) under ice-bath and stirred for Ih at room temperature under N2 atmosphere. To the resultant mixture was added 45g (220mmols) of aminourea 3 in 3 portions (3mins) at room temperature and stirred at 80 0C for Ih
References:
WO2009/75890,2009,A2 Location in patent:Page/Page column 36-37