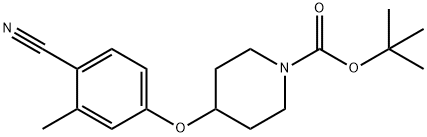
tert-butyl 4-(4-cyano-3-Methylphenoxy)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(4-cyano-3-Methylphenoxy)piperidine-1-carboxylate
- CAS Number:1164178-34-0
- Molecular formula:C18H24N2O3
- Molecular Weight:316.39

109384-19-2
420 suppliers
$5.00/1g
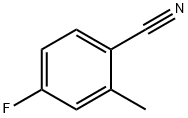
147754-12-9
290 suppliers
$5.00/1g

1164178-34-0
10 suppliers
inquiry
Yield:1164178-34-0 92%
Reaction Conditions:
Stage #1: t-butyl 4-hydroxy piperidine-1-carboxylatewith potassium tert-butylate in tert-butyl methyl ether; for 1 h;Reflux;
Stage #2: 4-fluoro-2-methylbenzonitrile in tert-butyl methyl ether; for 7 h;Reflux;Reagent/catalyst;Solvent;
Steps:
1.b 1)
a)
1.35 g 4-Fluoro-2-methyl-benzonitrile, dissolved in DMF, was added to 2.11 g 4-Hydroxy-piperidine-1-carboxylic acid tert-butyl ester and 0.6 g sodium hydride in 30 mL DMF.
The mixture was stirred at room temperature (rt) until the reaction was complete.
The reaction was quenched with water.
The aqueous layer was extracted with ethylacetate (AcOEt) or methyl tert. butyl ether (MTB ether).
The combined organic layers were washed with brine, dried and concentrated to to give 2.6 g (yield 81%) 4-(4-Cyano-3-methyl-phenoxy)-piperidine-1-carboxylic acid tert-butyl ester. Mass: (C18H24N2O3): calcd. 316, found 261 [M+H-t(C4H9)]+
References:
US2013/12711,2013,A1 Location in patent:Paragraph 0078