
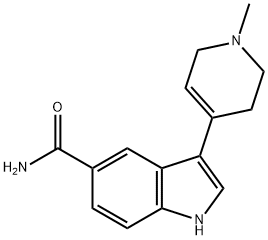
3-(1-METHYL-1,2,3,6-TETRAHYDRO-4-PYRIDINYL)-1H-INDOLE-5-CARBONITRILE synthesis
- Product Name:3-(1-METHYL-1,2,3,6-TETRAHYDRO-4-PYRIDINYL)-1H-INDOLE-5-CARBONITRILE
- CAS Number:116480-60-5
- Molecular formula:C15H15N3
- Molecular Weight:237.3
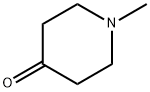
1445-73-4
466 suppliers
$15.00/25g
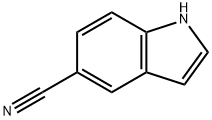
15861-24-2
436 suppliers
$10.00/5g

116480-60-5
16 suppliers
$43.79/250mg
Yield:116480-60-5 48.7%
Reaction Conditions:
with pyrrolidine in ethanol at 80; for 44 h;
Steps:
13 3-(1-Methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indole-5-carbonitrile (49)
To an argon-purged round bottom flask fitted with a magnetic stirbar containing an orange solution of 5-cyanoindole (48) (250 mg, 1.76 mmol) dissolved in absolute ethanol (10 mL) were added 1-methyl-4-piperidone (0.43 mL, 3.50 mmol) and pyrrolidine (0.44 mL, 5.27 mmol). The reaction vessel was fitted with a condenser and transferred to an oil bath preheated to 80° C. The reaction was stirred at this temperature for 44 hrs. As no starting material remained (TLC 5% 2M NH3 in methanol/95% CH2Cl2) the reaction was cooled to room temperature followed by additional cooling in the fridge. As no precipitate formed, the reaction was concentrated under reduced pressure to afford an orange oil. The oil was redissolved in ethanol (20 mL) and the solvent removed under reduced pressure. This was repeated once more, and then the final residue was treated with ethanol and left in the fridge for 2 hrs. The precipitate which formed was collected by vacuum filtration and washed with hexanes (205 mg of pale yellow solid, compound 49, 48.7%) 1H NMR (DMSO) δ 11.90 (br s, NH), 8.51 (s, 1H), 7.80 (s, 1H), 7.77-7.74 (d, J=8.7 Hz, 1H), 7.68-7.65 (d, J=8.1 Hz, 1H), 6.41 (s, 1H), 3.53 (s, 2H), 3.27-3.26 (d, J=2.4 Hz, 2H), 2.79-2.77 (d, J=4.5 Hz, 2H), 2.72-2.71 (d, J=1.5 Hz, 3H).
References:
US2006/258721,2006,A1 Location in patent:Page/Page column 52
116480-55-8
0 suppliers
inquiry

116480-60-5
16 suppliers
$43.79/250mg