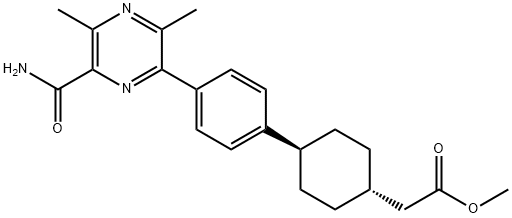
Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans- synthesis
- Product Name:Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans-
- CAS Number:1166831-68-0
- Molecular formula:C22H27N3O3
- Molecular Weight:381.47

1166828-19-8
15 suppliers
inquiry
![2-[(1r,4r)-4-[4-(tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]cyclohexyl]acetate](/CAS/GIF/701232-69-1.gif)
701232-69-1
34 suppliers
$55.00/100mg
![Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans-](/CAS/20150408/GIF/1166831-68-0.gif)
1166831-68-0
8 suppliers
inquiry
Yield:1166831-68-0 100%
Reaction Conditions:
with potassium phosphate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,2-dimethoxyethane;ethanol;water at 80; for 16 h;
Steps:
1.8
Intermediate 1-8: methyl 2-(( ls,4s)-4-(4-(6-carbamoyl-3.,5-dimethylpyrazin-2- vDphenvDcyclohexyDacetateA solution of 6-chloro-3,5-dimethylpyrazine-2-carboxamide (see Intermediate 21-4) (3.15 g, 16.97 mmol), methyl 2-((ls,4s)-4-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2- yl)phenyl)cyclohexyl)acetate (see Intermediate 64-8) (6.08 g, 16.97 mmol) and tripotassium phosphate (4.32 g, 20.37 mmol) in DME (120 mL), ethanol (75 mL) and water (30.0 mL) were degassed before addition of (l,l'-bis(diphenylphosphino)ferrocene)- dichloropalladium(II) (0.698 g, 0.85 mmol). The reaction mixture was heated to 80 0C, under nitrogen, and left to stir overnight for 16 hrs. The reaction mixture was allowed to cool to room temperature and then evaporated. The crude product was partitioned between water (250 mL) and EtOAc (250 mL). The catalyst was filtered off from the biphasic mixture. The organic phase was separated and washed with brine (100 mL), dried (Na2SO4) and evaporated. The crude product was purified by flash silica chromatography, elution gradient 5 to 90% EtOAc in isohexane on 330g silicyle column. Pure fractions were evaporated to dryness to afford the title compound (6.47 g, 100 %) as a yellow solid.[ 1H NMR (400.132 MHz, DMSO) δ 1.10 - 1.21 (2H, m), 1.46 - 1.56 (2H, m), 1.73 - 1.86 (5H, m), 2.25 (2H, d), 2.58 (3H, s ), 2.73 (3H, s), 3.28 (IH, s), 3.60 (3H, s), 7.35 (2H, d), 7.58 (IH, s), 7.64 (2H, d), 7.97 (IH, s); HPLC tR= 2.53 min.]
References:
WO2009/81195,2009,A1 Location in patent:Page/Page column 169
![Butanoic acid, 2-[[(2S)-2-[[(1,1-dimethylethoxy)carbonyl]amino]-1-oxopropyl]amino]-3-oxo-, ethyl ester](/CAS/20210305/GIF/1166827-50-4.gif)
1166827-50-4
0 suppliers
inquiry
![Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans-](/CAS/20150408/GIF/1166831-68-0.gif)
1166831-68-0
8 suppliers
inquiry

1166827-48-0
9 suppliers
inquiry
![Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans-](/CAS/20150408/GIF/1166831-68-0.gif)
1166831-68-0
8 suppliers
inquiry
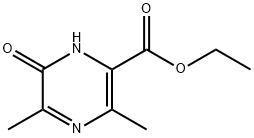
1166827-49-1
11 suppliers
inquiry
![Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans-](/CAS/20150408/GIF/1166831-68-0.gif)
1166831-68-0
8 suppliers
inquiry
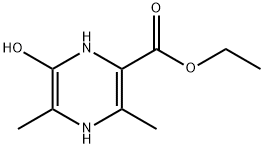
1166831-64-6
0 suppliers
inquiry
![Cyclohexaneacetic acid, 4-[4-[6-(aMinocarbonyl)-3,5-diMethyl-2-pyrazinyl]phenyl]-, Methyl ester, trans-](/CAS/20150408/GIF/1166831-68-0.gif)
1166831-68-0
8 suppliers
inquiry