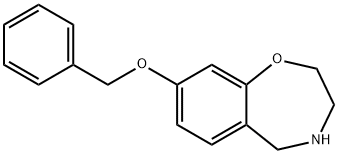
1,4-Benzoxazepine, 2,3,4,5-tetrahydro-8-(phenylmethoxy)- synthesis
- Product Name:1,4-Benzoxazepine, 2,3,4,5-tetrahydro-8-(phenylmethoxy)-
- CAS Number:1167416-19-4
- Molecular formula:C16H17NO2
- Molecular Weight:255.31
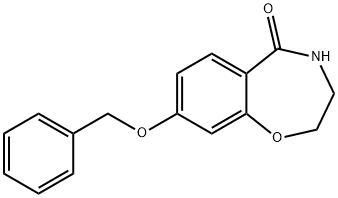
1167416-18-3
0 suppliers
inquiry

1167416-19-4
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 8-[(phenylmethyl)oxy]-3,4-dihydro-1,4-benzoxazepin-5(2H)-onewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 60; for 3.08333 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;water at 0;
Steps:
28
Preparation 28: 8-[(Phenylmethyl)oxy]-2,3,4,5-tetrahydro-1,4-benzoxazepine. 1 M Lithium aluminium hydride (62.4 ml, 62.4 mmol) in THF was added under argon to a stirred solution of 8-[(phenylmethyl)oxy]-3,4-dihydro-1 ,4-benzoxazepin-5(2H)- one (Preparation 27) (8.39 g, 31.2 mmol) in THF (150 ml) with ice bath cooling over 5 minutes. The resulting solution was heated with stirring at 60 0C for 3 hours. The reaction was cooled and carefully quenched by slow addition with ice cooling of 150ml of 2M sodium hydroxide followed by 150ml of EtOAc. The organics were separated and aqueous extracted with 100ml of EtOAc. The combined organic extracts were dried (magnesium sulphate) and evaporated to give the title compound (7.53g) as a light coloured oil. MS (ES) C16H17NO2 requires 255; found 256 [M+H]+.
References:
WO2009/80725,2009,A1 Location in patent:Page/Page column 45
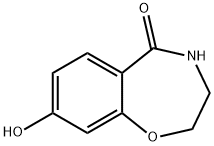
50502-78-8
2 suppliers
inquiry

1167416-19-4
0 suppliers
inquiry
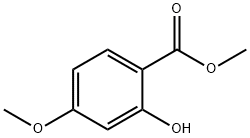
5446-02-6
178 suppliers
$25.00/5g

1167416-19-4
0 suppliers
inquiry
![Benzoic acid, 2-[2-[[(1,1-dimethylethoxy)carbonyl]amino]ethoxy]-4-methoxy-, methyl ester](/CAS/20200611/GIF/402933-40-8.gif)
402933-40-8
0 suppliers
inquiry

1167416-19-4
0 suppliers
inquiry
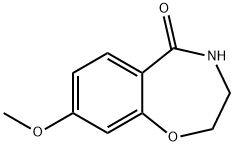
5755-00-0
5 suppliers
inquiry

1167416-19-4
0 suppliers
inquiry