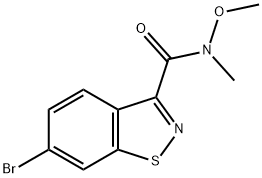
1,2-Benzisothiazole-3-carboxamide, 6-bromo-N-methoxy-N-methyl- synthesis
- Product Name:1,2-Benzisothiazole-3-carboxamide, 6-bromo-N-methoxy-N-methyl-
- CAS Number:1171245-18-3
- Molecular formula:C10H9BrN2O2S
- Molecular Weight:301.16
![6-Bromobenzo[d]isothiazole-3-carboxylic acid](/CAS/GIF/677304-75-5.gif)
677304-75-5
71 suppliers
$45.00/25mg

6638-79-5
555 suppliers
$6.00/25g

1171245-18-3
0 suppliers
inquiry
Yield:1171245-18-3 91%
Reaction Conditions:
with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
20.3
Step 3: DIEA (11.7 mL, 67.4 mmol) and HBTU (7.03 g, 18.5 mmol) were added to a solution of 6-bromobenzo[d]isothiazole-3-carboxylic acid (4.35 g, 16.9 mmol) and N,O-dimethylhydroxylamine hydrochloride (2.14 g, 21.9 mmol) in DMF (100 mL). The reaction was stirred at room temperature for 2 hours. The mixture was partitioned between water and EtOAc. The organic layer was washed with aqueous NaHCO3 solution and brine, dried and concentrated. The residue was purified by column chromatography (hexane:EtOAc, 3:1) to give 6-bromo-N-methoxy-N-methylbenzo[d]isothiazole-3- carboxamide (4.60 g, 91%) as a solid. 1H NMR (CDCl3, 400 MHz) δ 8.12 (m, 2H), 8.59 (d, J = 8.8 Hz, IH), 3.83 (s, 3H), 3.49 (s, 3H).
References:
WO2009/89462,2009,A1 Location in patent:Page/Page column 81