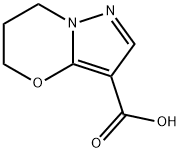
6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylic acid synthesis
- Product Name:6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylic acid
- CAS Number:1173003-61-6
- Molecular formula:C7H8N2O3
- Molecular Weight:168.15
![Ethyl 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylate](/CAS/GIF/1173003-60-5.gif)
1173003-60-5
14 suppliers
inquiry
![6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylic acid](/CAS/GIF/1173003-61-6.gif)
1173003-61-6
30 suppliers
$45.00/10mg
Yield:1173003-61-6 98%
Reaction Conditions:
Stage #1: 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylic acid ethyl esterwith water;lithium hydroxide in tetrahydrofuran;ethanol at 60; for 48 h;
Stage #2: with hydrogenchloride in water; pH=2;
Steps:
6,7-Dihydro-5H-pyrazolo[5,l-£] [l,3]oxazine-3-carboxylic acid[00360] A solution of 6,7-dihydro-5H-pyrazolo[5,l-6][l ,3]oxazine-3-carboxylic acid ethyl ester(9.67 g, 49.3 mmol) in tetrahydrofuran (150 mL), ethanol (50 mL) and IM of lithium hydroxide in water
References:
WO2009/89057,2009,A1 Location in patent:Page/Page column 56-57