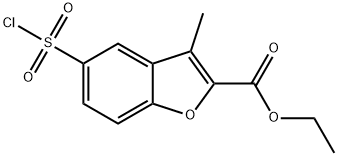
Ethyl 5-(chlorosulfonyl)-3-methyl-1-benzofuran-2-carboxylate synthesis
- Product Name:Ethyl 5-(chlorosulfonyl)-3-methyl-1-benzofuran-2-carboxylate
- CAS Number:1173083-97-0
- Molecular formula:C12H11ClO5S
- Molecular Weight:302.73
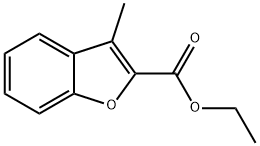
22367-82-4
87 suppliers
$142.00/1g

1173083-97-0
11 suppliers
$90.00/50mg
Yield:1173083-97-0 39%
Reaction Conditions:
with chlorosulfonic acid in chloroform at 20;Cooling with ice;
Steps:
2-benzoyl-3-methylbenzofuran-5-sulfonyl chloride (10g)
General procedure: To a solution of 9g (1.70g, 7.20mmol) in chloroform (30mL) cooled with an ice-water bath, the solution of chlorosulfonic acid (4.19g, 36.0mmol) in chloroform (10mL) was dropped in slowly. After the addition, the reactant was stirred at room temperature for 48 h. The reaction mixture was poured into ice-water (80mL) and the aqueous phase was extracted with dichloromethane (30mL) twice. The organic phase combined was washed with brine, dried with sodium sulfate anhydrous and then condensed under reduced pressure to give a yellow solid. Recrystallization of the crude product with cyclohexzane afforded 0.98g yellow solid, the yield was 41%.
References:
Yang, Li;Lei, Hua;Mi, Cheng-Gen;Liu, Huan;Zhou, Tian;Zhao, Ying-Lan;Lai, Xiao-Yun;Li, Zi-Cheng;Song, Hang;Huang, Wen-Cai [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 18,p. 5389 - 5392] Location in patent:supporting information; experimental part
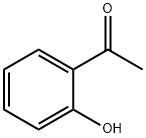
118-93-4
547 suppliers
$10.00/25g

1173083-97-0
11 suppliers
$90.00/50mg
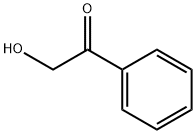
582-24-1
239 suppliers
$8.00/5g

1173083-97-0
11 suppliers
$90.00/50mg