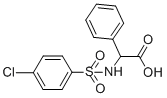
2-([(4-CHLOROPHENYL)SULFONYL]AMINO)-2-PHENYLACETIC ACID synthesis
- Product Name:2-([(4-CHLOROPHENYL)SULFONYL]AMINO)-2-PHENYLACETIC ACID
- CAS Number:117309-47-4
- Molecular formula:C14H12ClNO4S
- Molecular Weight:325.77
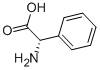
2935-35-5
400 suppliers
$5.00/10g
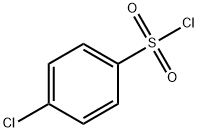
98-60-2
328 suppliers
$5.00/5g
![2-([(4-CHLOROPHENYL)SULFONYL]AMINO)-2-PHENYLACETIC ACID](/CAS/GIF/117309-47-4.gif)
117309-47-4
21 suppliers
$118.00/500mg
Yield:-
Reaction Conditions:
in water;acetone at 20; for 24 h;Alkaline conditions;
Steps:
3 4.1.1 General procedure for the synthesis of substituted sulfonamides using different aminoacids (14-21 and 22-35)
General procedure: L-isomers of different amino acids were used for the synthesis and in 1H NMR spectra it was found that only one isomer was formed indicating that there is retention in configuration. The substituted aromatic or aliphatic sulfonylchlorides (1-13, 58, 59) (0.0143mol, 1 equivalent) were dissolved in the acetone (2ml). The mixture was added to water (2ml) containing glycine/phenylglycine/phenylalanine (0.0143mol, 1 equivalent) and sodiumbicarbonate (0.0429mol, 3 equivalent). The final reaction mixture was stirred at room temperature (rt) for 24h. The reaction was monitored by TLC using ethylacetate and methanol (8:2) as the mobile phase. The acetone was evaporated under reduced pressure and reaction mixture was neutralized with 1N HCl to get the product. The product was filtered and recrystallized with methanol to get the pure compound [34].
References:
Kumar, Devendra;Gupta, Sukesh K.;Ganeshpurkar, Ankit;Gutti, Gopichand;Krishnamurthy, Sairam;Modi, Gyan;Singh, Sushil K. [European Journal of Medicinal Chemistry,2018,vol. 150,p. 87 - 101]