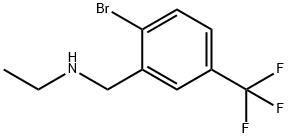
2-BROMO-N-ETHYL-5-(TRIFLUOROMETHYL)-BENZENEMETHANAMINE synthesis
- Product Name:2-BROMO-N-ETHYL-5-(TRIFLUOROMETHYL)-BENZENEMETHANAMINE
- CAS Number:1175526-32-5
- Molecular formula:C10H11BrF3N
- Molecular Weight:282.1
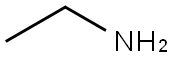
75-04-7
381 suppliers
$10.00/20mg

102684-91-3
181 suppliers
$7.00/1g

1175526-32-5
1 suppliers
inquiry
Yield:1175526-32-5 67%
Reaction Conditions:
Stage #1: ethylamine;2-bromo-5-(trifluoromethyl)benzaldehydewith sodium cyanoborohydride;acetic acid in tetrahydrofuran;methanol at 20;
Stage #2: with sodium hydrogencarbonate in dichloromethane;
Steps:
2 PREPARATION 2
N-[2-Bromo-5-(trifluoromethyl)benzyl]ethanamme
2-Bromo-5-(trifluoromethyl)benzaldehyde (20.0 g, 79.0 mmol) was dissolved in 300 ml methanol.
Ethylamine (2.0 M solution in tetrahydrofuran, 60 ml, 120 mmol) was added followed by the portion-wise addition of sodium cyanoborohydride (7.50 g, 120 mmol).
Acetic acid (8.0 ml, 140 mmol) was added drop-wise and the reaction mixture was stirred overnight at room temperature.
The organics were evaporated under reduced pressure and the mixture was partitioned between dichloromethane and water.
The aqueous phase was extracted twice with dichloromethane and the combined organics were washed with 4% w/v sodium bicarbonate solution, dried over sodium sulphate, filtered and evaporated under reduced pressure to give 15.0 g (53.2 mmol, 67%) of the title compound as an oil.
Purity 100%.
1H NMR (300 MHz, CHLOROFORM-d) δ ppm 7.70 (1 H, s), 7.66 (1 H, d, J=8.2 Hz), 7.37 (1 H, dd, J=8.2, 1.4 Hz), 3.91 (2 H, s), 2.71 (2 H, q, J=7.1 Hz), 1.68 (1 H, br. s.), 1.17 (3 H, t, J=7.1 Hz).
UPLC/MS (3 min) retention time 0.95 min.
LRMS: m/z 282, 284 (M+1).
References:
EP2548876,2013,A1 Location in patent:Paragraph 0204-0208