
(S)-1-((3AR,4R,6AS)-5-BENZYL-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROL-4-YL)-ETHANE-1,2-DIOL synthesis
- Product Name:(S)-1-((3AR,4R,6AS)-5-BENZYL-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROL-4-YL)-ETHANE-1,2-DIOL
- CAS Number:117781-06-3
- Molecular formula:C16H23NO4
- Molecular Weight:293.3581
![(3AR,4R,6AS)-5-BENZYL-4-((S)-2,2-DIMETHYL-[1,3]DIOXOLAN-4-YL)-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROLE](/CAS/GIF/117770-02-2.gif)
117770-02-2
7 suppliers
inquiry
![(S)-1-((3AR,4R,6AS)-5-BENZYL-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROL-4-YL)-ETHANE-1,2-DIOL](/CAS/GIF/117781-06-3.gif)
117781-06-3
7 suppliers
inquiry
Yield:117781-06-3 52%
Reaction Conditions:
with acetic acid in water at 50; for 48 h;
Steps:
4.2.7 (S)-1-((3aS,4S,6aR)-5-Benzyl-2,2-dimethyltetrahydro-4H-[1,3]dioxolo[4,5-c]pyrrol-4-yl)ethane-1,2-diol (24)
22 (1.4g, 4.3mmol) was stirred in 80% 21 aqueous acetic acid (20mL) at 50°C for 48h. After cooling to room temperature, a saturated aqueous solution of 92 NaHCO3 was added to neutralize the acetic acid, followed by extraction with ethyl acetate (3×). The combined organic layers were dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography (=4cm, h=15cm, V=30mL, ethyl acetate, Rf=0.29) to give 93 24 (0.65g, 2.2mmol, 52%) as colorless oil. Specific rotation: [α]20D [α]D20 =+22.7 (c=3.3, CH3OH); 1H NMR (DMSO-d6): δ [ppm]=1.23 (s, 3H, C(CH3)2), 1.45 (s, 3H, C(CH3)2), 2.53 (dd, J=11.4/3.2Hz, 1H, NCH2CH), 2.99 (dd, J=11.4/5.3Hz, 1H, NCH2CH), 3.03 (dd, J=4.9/1.4Hz, 1H, NCH), 3.35-3.41 (m, 1H, CH2OH), 3.45-3.56 (m, 2H, CH2OH (1H), CHOH), 3.79 (d, J=13.4Hz, 1H, NCH2Ph), 4.05 (d, J=13.4Hz, 1H, NCH2Ph), 4.48-4.59 (m, 3H, NCH2CHCH, CHOH, CH2OH), 4.62 (dd, J=6.1/1.6Hz, 1H, NCH2CHCH), 7.18-7.35 (m, 5H, Harom.); 13C NMR (DMSO-d6): δ [ppm]=24.4 (1C, C(CH3)2), 27.1 (1C, C(CH3)2), 58.3 (1C, NCH2CH), 59.8 (1C, NCH2Ph), 63.2 (1C, CH2OH), 71.0 (1C, NCH), 71.7 (1C, CHOH), 80.0 (1C, NCH2CH), 83.0 (1C, NCH2CHCH), 110.8 (1C, C(CH3)2), 126.7 (1C, C-4′phenyl), 128.1 (2C, C-3′phenyl, C-5′phenyl), 128.4 (2C, C-2′phenyl, C-6′phenyl), 139.9 (1C, C-1′phenyl); IR (neat): ν ν [cm-1]=3383, 2986, 2932, 2866, 1454, 1373, 1204, 1161, 1042, 864, 698; HRMS (m/z): [M+H]+ calcd for C16H24NO4: 294.1700, found: 294.1712; HPLC (method 1): tR=12.0, purity 96.3%.
References:
Kalinin, Dmitrii V.;Agoglitta, Oriana;Van de Vyver, Hélène;Melesina, Jelena;Wagner, Stefan;Riemann, Burkhard;Sch?fers, Michael;Sippl, Wolfgang;L?ffler, Bettina;Holl, Ralph [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 10,p. 1997 - 2018]
![(2,2-DIMETHYL-[1,3]DIOXOLAN-4-YL)-(5-HYDROXYMETHYL-2,2-DIMETHYL-[1,3]DIOXOLAN-4-YL)-METHANOL](/CAS/GIF/3969-61-7.gif)
3969-61-7
33 suppliers
$62.50/1g
![(S)-1-((3AR,4R,6AS)-5-BENZYL-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROL-4-YL)-ETHANE-1,2-DIOL](/CAS/GIF/117781-06-3.gif)
117781-06-3
7 suppliers
inquiry
![METHANESULFONIC ACID (4S,5S)-5-[(S)-((R)-2,2-DIMETHYL-[1,3]DIOXOLAN-4-YL)-METHANESULFONYLOXY-METHYL]-2,2-DIMETHYL-[1,3]DIOXOLAN-4-YLMETHYL ESTER](/CAS/GIF/7115-24-4.gif)
7115-24-4
1 suppliers
inquiry
![(S)-1-((3AR,4R,6AS)-5-BENZYL-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROL-4-YL)-ETHANE-1,2-DIOL](/CAS/GIF/117781-06-3.gif)
117781-06-3
7 suppliers
inquiry
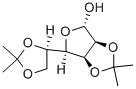
14131-84-1
230 suppliers
$6.00/1g
![(S)-1-((3AR,4R,6AS)-5-BENZYL-2,2-DIMETHYL-TETRAHYDRO-[1,3]DIOXOLO[4,5-C]PYRROL-4-YL)-ETHANE-1,2-DIOL](/CAS/GIF/117781-06-3.gif)
117781-06-3
7 suppliers
inquiry