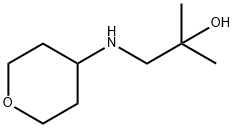
2-Methyl-1-(tetrahydro-pyran-4-ylamino)-propan-2-ol synthesis
- Product Name:2-Methyl-1-(tetrahydro-pyran-4-ylamino)-propan-2-ol
- CAS Number:1178195-37-3
- Molecular formula:C9H19NO2
- Molecular Weight:173.25
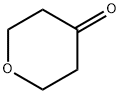
29943-42-8
427 suppliers
$5.00/1g
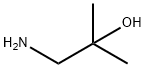
2854-16-2
233 suppliers
$14.00/1g

1178195-37-3
8 suppliers
inquiry
Yield:1178195-37-3 2.8 g
Reaction Conditions:
Stage #1: Tetrahydro-4H-pyran-4-one;1-Amino-2-methyl-propan-2-olwith acetic acid in methanol at 20; for 0.5 h;Inert atmosphere;
Stage #2: with methanol;sodium cyanoborohydride for 15 h;Inert atmosphere;
Steps:
14.1 Step 14.1 : (preparation of a compound of formula (XI)) 2-Methyl-1 -((tetrahydro-2H-pyran-4-yl)amino)propan-2-ol
Step 14.1 : (preparation of a compound of formula (XI)) 2-Methyl-1 -((tetrahydro-2H-pyran-4-yl)amino)propan-2-ol 2.75 g of 1-amino-2-methylpropan-2-ol are dissolved in 20 ml of MeOH. 1 .71 ml of glacial acetic acid are added dropwise under an inert atmosphere. 2 g of tetrahydropyran- 4-one in 10 ml of MeOH are added, the mixture is stirred for 30 minutes at room temperature and 1.98 g of sodium cyanoborohydride are added. The resulting mixture is stirred under an inert atmosphere for 15 hours. The reaction medium is poured into saturated aqueous NaHC03 solution (100 ml). The resulting mixture is stirred for 30 minutes and then extracted with 4 times 100 ml of DCM. The organic phase is dried over Na2S04, filtered and evaporated without heating. The MeOH contained in the aqueous phase is evaporated off and the extraction, drying, filtration and evaporation operation is repeated 3 times. 2.8 g of 2-methyl-1 -((tetrahydro-2H-pyran-4-yl)amino)propan-2-ol are recovered in total.
References:
WO2013/111106,2013,A1 Location in patent:Page/Page column 97