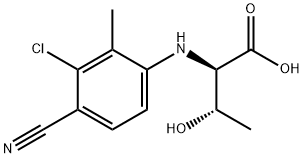
(2R,3S)-2-((3-chloro-4-cyano-2-methylphenyl)amino)-3-hydroxybutanoic acid synthesis
- Product Name:(2R,3S)-2-((3-chloro-4-cyano-2-methylphenyl)amino)-3-hydroxybutanoic acid
- CAS Number:1182366-63-7
- Molecular formula:C12H13ClN2O3
- Molecular Weight:268.7
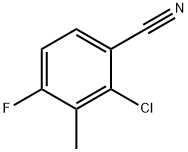
796600-15-2
134 suppliers
$10.00/250mg

632-20-2
434 suppliers
$6.00/5g

1182366-63-7
4 suppliers
inquiry
Yield:1182366-63-7 54%
Reaction Conditions:
Stage #1: 2-chloro-4-fluoro-3-methylbenzonitrile;D-Threoninewith potassium carbonate in dimethyl sulfoxide at 75; for 24 h;
Stage #2: with citric acid in water;dimethyl sulfoxide at 20;
Steps:
1.1a
Example 1; 2-chloro-4-((1R,2S)-2-hydroxy-1-(5-phenyl-1,3,4-oxadiazol-2-yl)propylamino)-3-methylbenzonitrile; Intermediate 1a; (2R,3S)-2-(3-chloro-4-cyano-2-methylphenylamino)-3-hydroxybutanoic acid; 2-chloro-4-fluoro-3-methylbenzonitrile (CAS 796600-15-2, 45g, 265.4 mmol) was mixed together with H-D-Thr-OH (37.92 g, 318.4 mmol) in DMSO (250 mL). K2CO3 (73.35 g, 530.7 mmol) was added to the reaction mixture and the reaction mixture stirred at 75° C. for 24 h. The reaction mixture was cooled to room temperature and poured slowly into a 10% citric acid solution and stirred for 10 min at room temperature. The solution was extracted with EtOAc several times to get the crude product. The crude product was chromatographed on silica gel with a gradient of hexanes/EtOAc and then with EtOAc, 100% to get the purified final product (39.0 g, 54%) 1H NMR (500 MHz, Acetone-d6, δ in ppm) 7.49 (d, J=9 Hz, 1H), 6.70 (d, J=9 Hz, 1H), 5.38 (d, J=10 Hz, 1H), 4.47 (d, J=6 Hz, 1H), 4.25 (m, 1H), 2.34 (s, 3H), 1.33 (d, J=6 Hz, 3H).
References:
US2009/253758,2009,A1 Location in patent:Page/Page column 28-29