[1,1'-Biphenyl]-3-carboxylic acid, 4-chloro-3'-fluoro- synthesis
- Product Name:[1,1'-Biphenyl]-3-carboxylic acid, 4-chloro-3'-fluoro-
- CAS Number:1183702-65-9
- Molecular formula:C13H8ClFO2
- Molecular Weight:250.65
Yield:1183702-65-9 67%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in ethanol;water;N,N-dimethyl-formamide at 100;Inert atmosphere;
Steps:
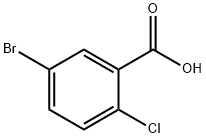
12 2-Chloro-5-(3-fluorophenyl)benzoic acid (intermediate 111(g))
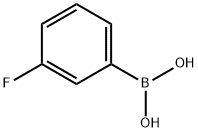
A solution of 3-fiuorophenyl boronic acid (500 mg, 3.5 mmol, 1.2 eq), 5-bromo-2- chlorobenzoic acid (700 mg, 3.0 mmol, 1.0 eq), Pd(PPh3)4 (687 mg, 0.60 mmol, 0.2 eq) and Na2C03 (2.52 g, 24 mmol, 8.0 eq) in a mixture of ethanol (5 mL), H20 (5mL) and DMF (20 mL) was stirred at 100°C under N2 overnight. The reaction was quenched by addition of diluted HCl (to pH 3) and the aqueous phase was extracted with EtOAc. The combined organic extracts were washed with brine, dried (Na2S04) filtered and evaporated in vacuo. The residue was purified by chromatography (petroleum ethenEtOAc 20:1 to 1 : 1) to give the title compound as a white solid (500 mg, 67 %). LC-MS : m/z 249.0, 251.0 [M-HV1H NMR (400 MHz, DMSO-d6) ? 13.58 (br s, 1H), 8.06 (d, J= 2.2 Hz, 1H), 7.88 (dd, J = 8.3, 2.4 Hz, 1H), 7.65 (d, J= 8.4 Hz, 1H), 7.63 - 7.50 (m, 3H), 7.26 (br t, J= 8.2 Hz, 1H)
References:
WO2013/37705,2013,A2 Location in patent:Page/Page column 91