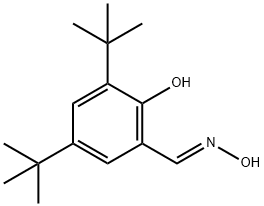
2,4-di-tert-butyl-6-[(E)-(hydroxyimino)methyl]phenol synthesis
- Product Name:2,4-di-tert-butyl-6-[(E)-(hydroxyimino)methyl]phenol
- CAS Number:118371-00-9
- Molecular formula:C15H23NO2
- Molecular Weight:249.3486
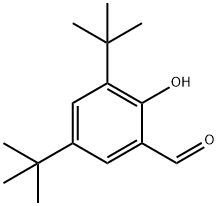
37942-07-7
372 suppliers
$5.00/5g
![2,4-di-tert-butyl-6-[(E)-(hydroxyimino)methyl]phenol](/CAS/20180703/GIF/118371-00-9.gif)
118371-00-9
5 suppliers
inquiry
Yield:118371-00-9 98%
Reaction Conditions:
with pyridine;hydroxylamine hydrochloride in ethanol at 80; for 2 h;
Steps:
1B.B1
[00296] Section IB. 5-Aryl Phenol Oxadiazole Ligands Scheme Bl; [00297] A reaction flask was charged with BB7 (1.0 g, 4.23 mmol), hydroxylamine hydrochloride (356 mg, 5.12 mmol), EtOH (8.0 mL), and pyridine (0.41 mL, 5.12 mmol). The reaction was heated at 8O°C for 2h. Ethanol was removed by rotary evaporation and the reaction mixture was taken up in Et2O / H2O. The layers were separated and the organic layer was washed with H2O, brine, and then dried over Na2SO4. Isolated 1.04 g, 98% yield, of 3,5-di-t-butyl-2-hydroxybenzaldehyde oxime as a white solid. 1H NMR (300 MHz, C6D6) δ 1.23 (s, 9H, 'Bu), 1.64 (s, 9H, tBu), 6.06 (s, 1H, NOH), 6.72 (d, 1H), 7.50 (d, 1H), 7.84 (s, 1H), 10.49 (s, 1H, OH). EPO
References:
WO2006/66126,2006,A2 Location in patent:Page/Page column 71