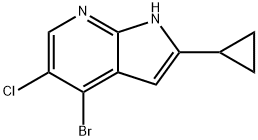
1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl-
- CAS Number:1187449-10-0
- Molecular formula:C10H8BrClN2
- Molecular Weight:271.54
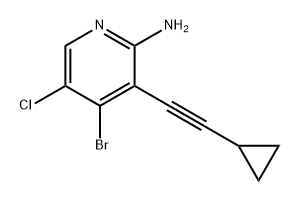
1187449-08-6
0 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl-](/CAS/GIF/1187449-10-0.gif)
1187449-10-0
1 suppliers
inquiry
Yield:1187449-10-0 51%
Reaction Conditions:
with potassium tert-butylate in N,N-dimethyl-formamide at 35; for 10.5 h;Inert atmosphere;
Steps:
17
Description 17 (D17); ^bromo-S-chloro^-cyclopropyl-I H-pyrrolo^.S-blpyridine; To a stirred solution of 4-bromo-5-chloro-3-(cyclopropylethynyl)-2-pyridinamine (D16) (4.10 g, 15.0 mmol) in N,N-dimethylformamide (36 ml.) was added potassium tert- butoxide (4.214 g, 38.00 mmol) under argon. Reaction mixture heated to 350C using an oil bath and allowed to stir for 10.5 h. Reaction mixture poured into water (300 ml_) and stirred in ether (400 ml_). Layers separated and aqueous portion extracted with ether (2 x 200 ml_). The combined extracts were dried over magnesium sulfate, filtered and solvent removed in vacuo yielding a pale brown solid. Solid recrystallised with ethyl acetate (50 ml_), solid filtered, washed and dried in oven yielding the title compound as an off-white solid (2.1 O g, 51 %).1H NMR: (300 MHz, CDCI3) δ 9.67 (s, 1 H), 8.16 (s, 1 H), 6.14 (s, 1 H), 2.08-2.00 (m, 1 H), 1.14-1.10 (m, 2H), 0.91-0.89 (m, 2H).
References:
WO2009/112473,2009,A1 Location in patent:Page/Page column 70
84249-14-9
362 suppliers
$6.00/1g
![1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl-](/CAS/GIF/1187449-10-0.gif)
1187449-10-0
1 suppliers
inquiry
6746-94-7
323 suppliers
$13.00/1g
![1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl-](/CAS/GIF/1187449-10-0.gif)
1187449-10-0
1 suppliers
inquiry

1187449-01-9
65 suppliers
$71.00/1g
![1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl-](/CAS/GIF/1187449-10-0.gif)
1187449-10-0
1 suppliers
inquiry
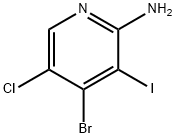
1187449-04-2
6 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine, 4-broMo-5-chloro-2-cyclopropyl-](/CAS/GIF/1187449-10-0.gif)
1187449-10-0
1 suppliers
inquiry