
2,6,7-Trioxa-1-borabicyclo[2.2.2]octan-3-one, 4-methyl- synthesis
- Product Name:2,6,7-Trioxa-1-borabicyclo[2.2.2]octan-3-one, 4-methyl-
- CAS Number:1188369-88-1
- Molecular formula:C5H7BO4
- Molecular Weight:141.92
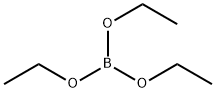
150-46-9
186 suppliers
$14.00/25mL
4767-03-7
379 suppliers
$6.00/25g
![2,6,7-Trioxa-1-borabicyclo[2.2.2]octan-3-one, 4-methyl-](/CAS/20210111/GIF/1188369-88-1.gif)
1188369-88-1
0 suppliers
inquiry
Yield:1188369-88-1 100%
Reaction Conditions:
in ethanol;
Steps:
3
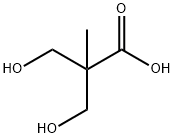
EXAMPLE 3 13.061 g of ethyl borate B(OC2H5)3 are added, in 75 ml of 95% ethanol, to 12 g of commercially available 2,2-bis(hydroxymethyl)propionic acid CH3C(CH2OH)2CO2H, with magnetic stirring. The clear solution obtained is evaporated under vacuum on a rotary evaporator and the drying is continued under a primary vacuum at 75° C. The syrupy mass is converted into a white solid which is isolated (quantitative yield) and corresponds to the formula CH3C[(CH2O-)2(CO2-)]B (IB-3). 6 g of this complex are dissolved in 25 ml of anhydrous dimethylformamide (DMF) and a clear solution is obtained. 2.46 g of potassium fluoride dried at 150° C. under a primary vacuum are added. KF, which is strictly insoluble in DMF, goes into solution rapidly so as to form the adduct K+{CH3C[(CH2O-)2(CO2-)BF}-.
References:
US2011/171112,2011,A1 Location in patent:Page/Page column 13
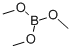
121-43-7
346 suppliers
$14.00/25mL

4767-03-7
379 suppliers
$6.00/25g
![2,6,7-Trioxa-1-borabicyclo[2.2.2]octan-3-one, 4-methyl-](/CAS/20210111/GIF/1188369-88-1.gif)
1188369-88-1
0 suppliers
inquiry