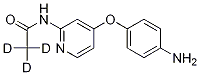
[4-(4-Aminophenoxy)(2-pyridyl)]-N-(methyl-d3)carboxamide synthesis
- Product Name:[4-(4-Aminophenoxy)(2-pyridyl)]-N-(methyl-d3)carboxamide
- CAS Number:1189975-18-5
- Molecular formula:C13H10D3N3O2
- Molecular Weight:246
Yield:1189975-18-5 92%
Reaction Conditions:
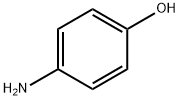
Stage #1: 4-amino-phenolwith potassium tert-butylate in N,N-dimethyl-formamide at 20; for 2 h;
Stage #2: 4-chloropyridinyl-2-(N-1',1',1'-trideuteromethylcarboxamide)with potassium carbonate in N,N-dimethyl-formamide at 80;
Steps:
1.2 2.
Preparation of 4-(4-aminophenoxy)-2-pyridine-(N-(methyl-d3)) carboxamide (5)
To dry DMF (100 mL) was added 4-aminophenol (9.54 g, 0.087 mol) and potassium tert-butoxide (10.3 g, 0.092 mol) in turn.
The color of the solution turned into deep brown.
After stirring at room temperature for 2 hours, to the reaction mixture was added 4-chloro-(N-methyl-d3)pyridine-2-carboxamide (3) (13.68 g, 0.079 mol) and anhydrous potassium carbonate (6.5 g, 0.0467 mol), then warmed up to 80° C. and stirred over night.
The reaction was completed by TLC detection.
The reaction mixture was cooled to room temperature, and poured into a mixed solution of ethyl acetate (150 mL) and saturated brine (150 mL).
The mixture was stirred and then stood for layering.
The aqueous phase was extracted with ethyl acetate (3*100 mL).
The extracted layers were combined, washed with saturated brine (3*100 mL) prior to drying over anhydrous sodium sulfate, and concentrated to afford 4-(4-aminophenoxy)-2-pyridine-(N-(methyl-d3))carboxamide (18.00 g, 92% yield) which was light yellow.
1H NMR (CDCl3, 300 MHz): 8.32 (d, 1H), 7.99 (br, 1H), 7.66 (s, 1H), 6.91-6.85 (m, 3H), 6.69 (m, 2H), 3.70 (br, s, 2H).
References:
US2013/12548,2013,A1 Location in patent:Paragraph 0124-0126
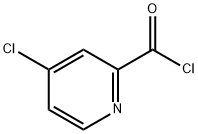
53750-66-6
91 suppliers
$60.00/250mg
![[4-(4-Aminophenoxy)(2-pyridyl)]-N-(methyl-d3)carboxamide](/CAS/GIF/1189975-18-5.gif)
1189975-18-5
8 suppliers
inquiry
98-98-6
603 suppliers
$5.00/10g
![[4-(4-Aminophenoxy)(2-pyridyl)]-N-(methyl-d3)carboxamide](/CAS/GIF/1189975-18-5.gif)
1189975-18-5
8 suppliers
inquiry
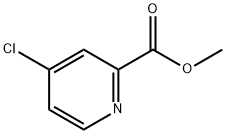
24484-93-3
399 suppliers
$6.00/5g
![[4-(4-Aminophenoxy)(2-pyridyl)]-N-(methyl-d3)carboxamide](/CAS/GIF/1189975-18-5.gif)
1189975-18-5
8 suppliers
inquiry
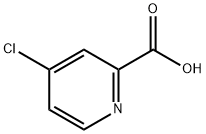
5470-22-4
302 suppliers
$7.00/5g
![[4-(4-Aminophenoxy)(2-pyridyl)]-N-(methyl-d3)carboxamide](/CAS/GIF/1189975-18-5.gif)
1189975-18-5
8 suppliers
inquiry