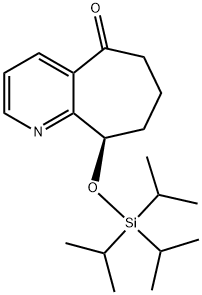
(R)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-onehydrochloride synthesis
- Product Name:(R)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-onehydrochloride
- CAS Number:1190363-45-1
- Molecular formula:C19H31NO2Si
- Molecular Weight:333.54
![(9R)-9-hydroxy-6,7,8,9-tetrahydrocyclohepta[b]pyridin-5-one](/CAS/20180531/GIF/1190363-44-0.gif)
1190363-44-0
65 suppliers
inquiry
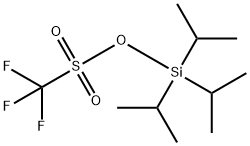
80522-42-5
255 suppliers
$12.00/1g
![(R)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-onehydrochloride](/CAS/20180703/GIF/1190363-45-1.gif)
1190363-45-1
83 suppliers
inquiry
Yield:1190363-45-1 73%
Reaction Conditions:
with triethylamine in dichloromethane;benzene at 0; for 1 h;
Steps:
(R)-9-(Triisopropylsilyloxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-one. In a 1L round-bottomed flask was (R)-9-hydroxy-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-one (19.14 g, 108 mmol) (azeotroped with dry benzene) in CH2Cl2 (300 mL) to give a tan solution. After cooling to 0° C., TIPS-OTf (29.3 mL, 108 mmol) and Et3N (30.1 mL, 216 mmol) were added via syringe, and the mixture was stirred at 0° C. for 1h. LCMS indicated complete conversion. Volatiles were stripped off and the residue was partitioned between NaHCO3 solution and EtOAc. The layers were separated and the organic layer was washed with brine, dried and concentrated to a tan oil (37 g). It was purified by FCC up to 20% EtOAc/hexane to afford the product as a white solid (26.3 g, 73% for two steps). MS(ESI)[M+H+]=334.37.
References:
US2009/258866,2009,A1 Location in patent:Page/Page column 31
![7,8-dihydro-5H-cyclohepta[b]pyridine-5,9(6H)-dione](/CAS/GIF/39713-40-1.gif)
39713-40-1
135 suppliers
$155.00/50mg
![(R)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-onehydrochloride](/CAS/20180703/GIF/1190363-45-1.gif)
1190363-45-1
83 suppliers
inquiry
605-38-9
189 suppliers
$14.00/5g
![(R)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-onehydrochloride](/CAS/20180703/GIF/1190363-45-1.gif)
1190363-45-1
83 suppliers
inquiry
![dimethyl 5,9-dihydroxy-7H-cyclohepta[b]pyridine-6,8-dicarboxylate](/CAS/20200119/GIF/39713-36-5.gif)
39713-36-5
17 suppliers
inquiry
![(R)-9-((triisopropylsilyl)oxy)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-5-onehydrochloride](/CAS/20180703/GIF/1190363-45-1.gif)
1190363-45-1
83 suppliers
inquiry