
(S)-4-Oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid synthesis
- Product Name:(S)-4-Oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid
- CAS Number:1190392-22-3
- Molecular formula:C8H8N2O3
- Molecular Weight:180.16
![methyl(S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylate](/CAS/20180527/GIF/1190392-23-4.gif)
1190392-23-4
20 suppliers
inquiry
![(S)-4-Oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid](/CAS/GIF/1190392-22-3.gif)
1190392-22-3
48 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: methyl [6(S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]]pyrimidine-6-carboxylatewith water;lithium hydroxide in tetrahydrofuran;methanol at 20; for 1 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water;
Steps:
B
Methyl [6(1?)-4-oxo-4,6,738-tetrahydropyrrolo[lJ2-a]pyrimidine-6-carboxyiate (9.95 g, 51.2 mmol) in tetrahydrofuran (60 mL), methanol (40 mL) and a solution of lithium hydroxide (3.32g, 77 mmol) in water (40 mL) was stirred at ambient temperature for 1 h. 2 N hydrochloric acid (38.5 mL) was added to neutralize the reaction mixture which was then directly purified by reverse phase HPLC (TMC Pro-Pac C18; 0-40% 0.1% trifluoroacetic acid in acetonitrile/0.1% trifluoroacetic acid in water gradient). The O-alkylation product was eluted fast The pure fractions were collected and lyophilized overnight to afford the title compound as a pale yellow solid. lH NMR (DMSO-ifc): δ 7.89 (d, J - 6.6 Hz, 1H), 6.24 (d, J - 6.6 Hz, 1H), 4.92 (dd, J = 10.0, 3.1 Hz, 1H), 3.12-2.99 (m, 2H), 2.52 (m, 1H), 2.11 (m, 1H). LC/MS 181.2 (M+l).
References:
WO2011/43942,2011,A1 Location in patent:Page/Page column 33-34
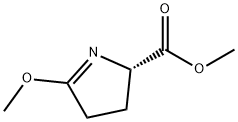
173142-47-7
13 suppliers
inquiry
![(S)-4-Oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid](/CAS/GIF/1190392-22-3.gif)
1190392-22-3
48 suppliers
inquiry
![5,8-Methanopyrrolo[2,1-b]quinazoline-1-carboxylic acid, 1,2,3,4a,5,8,8a,9-octahydro-9-oxo-, methyl ester, (1S)-](/CAS/20210305/GIF/1190709-67-1.gif)
1190709-67-1
0 suppliers
inquiry
![(S)-4-Oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxylic acid](/CAS/GIF/1190392-22-3.gif)
1190392-22-3
48 suppliers
inquiry