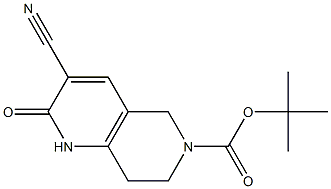
tert-butyl 3-cyano-2-oxo-1,2,5,6,7,8-hexahydro-1,6-naphthyridine-6-carboxylate synthesis
- Product Name:tert-butyl 3-cyano-2-oxo-1,2,5,6,7,8-hexahydro-1,6-naphthyridine-6-carboxylate
- CAS Number:1190440-61-9
- Molecular formula:C14H17N3O3
- Molecular Weight:275.3031
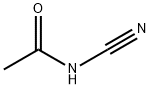
5634-51-5
2 suppliers
inquiry

79099-07-3
543 suppliers
$5.00/5g
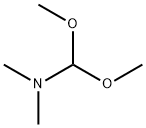
4637-24-5
588 suppliers
$5.00/25g

1190440-61-9
6 suppliers
inquiry
Yield:1190440-61-9 32%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;N,N-dimethyl-formamide dimethyl acetal in toluene at 20 - 105;
Stage #2: acetylcyanamidewith sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20;
Stage #3: with hydrogenchloride in water;N,N-dimethyl-formamide;mineral oil at 0;
Steps:
11
To a stirred solution of tert-butyl 4-oxopiperidine-l-carboxylate (20.0 g, 100.5 mmol) in toluene (80 ml) at room temperature was added DMF.DMA (12.56g, 105.5 mmol) and the reaction mixture was heated to 105 0C and stirred at that temperature for 16 h. After cooling, volatiles were removed under reduced pressure. The resulting pale red oil was dissolved in DMF (400 ml), cooled to 0 0C before cyanoacetamide (8.86 g, 105.5 mmol) and NaH (60 % in oil, 7.23 g, 180.9 mmol) were added. The reaction mixture was stirred at room temperature for 16 h. After cooling to 0 0C, water (50 ml) was added and the mixture was acidified to pH 4 with 2N HCl. The solids were isolated by filtration, washed with water, heptane, dried at reduced pressure to give the title compound (8.78 g, 32 %) as brown solid.LCMS data: Calculated MH+ (276); Found 100% (MH+) m/z 276, Rt = 1.48 min.1H NMR (400 MHz, DMSO-de) δ ppm 12.52 (1 H, br. s.), 8.03 (1 H, s), 4.23 (2 H, s), 3.54 (2H, t, J=5.7 Hz), 2.64 (2 H, t, J=5.7 Hz), 1.41 (9 H, s).
References:
WO2009/121812,2009,A1 Location in patent:Page/Page column 88

79099-07-3
543 suppliers
$5.00/5g

1190440-61-9
6 suppliers
inquiry