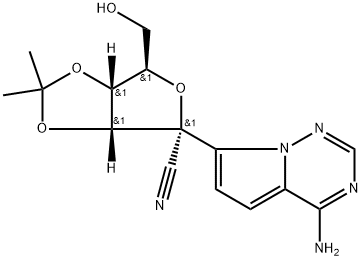
Remdesivir N-2 synthesis
- Product Name:Remdesivir N-2
- CAS Number:1191237-80-5
- Molecular formula:C15H17N5O4
- Molecular Weight:331.33
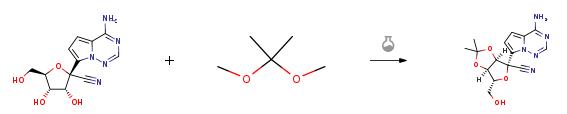
5.62g of compound GS-441524 was dissolved in 30mL of acetone, 11.50mL of 2,2-dimethoxypropane and 1.34mL of sulfuric acid were added, stirred at 45°C for half an hour, cooled to 25°C, and the organic solvent was removed by rotary evaporation. It was extracted with 100 mL of ethyl acetate and 100 mL of saturated sodium bicarbonate aqueous solution, and the extraction was repeated three times. The ethyl acetate layers were combined, dried by adding anhydrous sodium sulfate, and filtered to remove sodium sulfate. The organic solvent was removed by rotary evaporation, and column chromatography was performed (eluent: petroleum ether/ethyl acetate (V/V)=1/2) to obtain 6.20 g of compound 5 (white solid, 97% yield).
1191237-69-0
326 suppliers
$8.00/1mg

77-76-9
389 suppliers
$9.00/5ml

1191237-80-5
101 suppliers
inquiry
Yield:1191237-80-5 96%
Reaction Conditions:
with sulfuric acid in acetone at 20; for 0.03 h;Temperature;Time;Solvent;
Steps:
1-9 Example 7
This example provides a new type of microchannel reactor for preparing key intermediates of remdesivir (3aR,4R,6R,6aR)-4-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuran[3,4-d]dioxazole-4-carbonitrile, as shown in figure 1, The specific synthesis method includes the following steps: Weigh (2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazine-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-nitrile 5.82g (20mmol, 1.0equiv), fully dissolved in acetone. Add 12.2 mL (100 mmol, 5.0 equiv) of 2,2-dimethoxypropane to prepare a 50 mL solution as material I; measure 3.0 mL (10M, 30 mmol, 1.5 equiv) of sulfuric acid as material II. Pump material I and material II at the same time, where the feed flow rate of material I is 2.0 mL/min, and the feed flow rate of material II is 0.12 mL/min. After mixing, they are transported to the reaction module for reaction. Among them, the pipe inner diameter of the reaction module is 1.0mm, length 5m, volume 3.9mL, residence time 1.8min, reaction temperature 20°C. The reaction was monitored by TLC. After the reaction was completed, the effluent was collected, added 5.8g NaHCO3 and 5.8mL water, stirred for 15min, distilled under reduced pressure, extracted with ethyl acetate and water and collected the organic phase, dried with anhydrous Na2SO4, Vacuum distillation and drying to obtain (3aR,4R,6R,6aR)-4-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-6-(hydroxymethyl)-2,2-dimethyltetrahydrofuran[3,4-d]dioxazole-4-carbonitrile 6.35g, yield 96%.
References:
CN112250696, 2021, A Location in patent:Paragraph 0037-0086
![7-Iodopyrrolo[2,1-f][1,2,4]triazin-4-amine](/CAS/20200611/GIF/1770840-43-1.gif)
1770840-43-1
268 suppliers
inquiry

1191237-80-5
101 suppliers
inquiry
![D-Ribofuranose, 1-C-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2,3,5-tris-O-(phenylmethyl)-](/CAS/20200611/GIF/1355049-94-3.gif)
1355049-94-3
46 suppliers
inquiry

1191237-80-5
101 suppliers
inquiry
![PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE](/CAS/GIF/159326-68-8.gif)
159326-68-8
327 suppliers
$10.00/250mg

1191237-80-5
101 suppliers
inquiry
16838-89-4
31 suppliers
inquiry

1191237-80-5
101 suppliers
inquiry