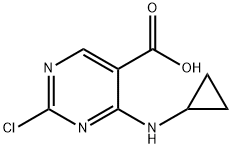
2-Chloro-4-(Cyclopropylamino)Pyrimidine-5-Carboxylic Acid synthesis
- Product Name:2-Chloro-4-(Cyclopropylamino)Pyrimidine-5-Carboxylic Acid
- CAS Number:1192711-37-7
- Molecular formula:C8H8ClN3O2
- Molecular Weight:213.62

1192711-36-6
8 suppliers
inquiry

1192711-37-7
25 suppliers
$109.00/100mg
Yield:1192711-37-7 64%
Reaction Conditions:
Stage #1: ethyl 2-chloro-4-(cyclopropylamino)pyrimidine-5-carboxylatewith water;lithium hydroxide in 1,4-dioxane at 20;
Stage #2: with hydrogenchloride in 1,4-dioxane;water; pH=2;
Steps:
5.5
Ethyl ester 1.5 (6.02g, 24 mmol) was diluted with 1,4-dioxane (26 mL) followed by aqueous lithium hydroxide (1.0 M, 26 mL, 26 mmol) and stirred at rt until all starting material had been converted to the carboxylic acid. The reaction was then diluted with water to a total volume of 100 mL and acidified to pH = 2 with 6 M HCl. The resulting suspension was then filtered and dried by aspiration giving 3.5 Ig of the carboxylic acid (64%). 1H NMR (DMSOd6, 400 MHz): δ 8.64 (d, IH), 8.74 (s, IH), 4.50 (m, IH), 2.31 (m, 2H), 2.03 (m, 2H), 1.72 (m, 2H).
References:
WO2009/131687,2009,A2 Location in patent:Page/Page column 102
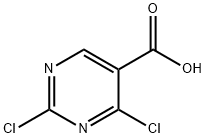
37131-89-8
144 suppliers
$65.00/250mg
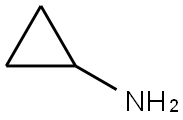
765-30-0
491 suppliers
$10.00/25g

1192711-37-7
25 suppliers
$109.00/100mg