
tert-butyl 2'-tert-butyl-7'-oxo-6',7'-dihydro-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-1-carboxylate synthesis
- Product Name:tert-butyl 2'-tert-butyl-7'-oxo-6',7'-dihydro-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-1-carboxylate
- CAS Number:1197815-67-0
- Molecular formula:C19H29N3O4
- Molecular Weight:363.45
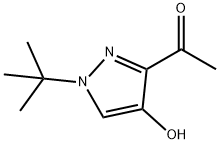
1197815-66-9
13 suppliers
inquiry

79099-07-3
543 suppliers
$5.00/5g
![tert-butyl 2'-tert-butyl-7'-oxo-6',7'-dihydro-2'H-spiro[piperidine-4,5'-pyrano[3,2-c]pyrazole]-1-carboxylate](/CAS/20130318/GIF/CB82574691.gif)
1197815-67-0
28 suppliers
$45.00/100mg
Yield:1197815-67-0 60%
Reaction Conditions:
Stage #1: 1-(1-(tert-butyl)-4-hydroxy-1H-pyrazol-3-yl)ethanone in methanol at 20; for 2 h;
Stage #2: N-tert-butyloxycarbonylpiperidin-4-one in methanol; for 24 h;Reflux;
Steps:
(A) Pyruvic aldehyde (2, 25 mmol) was added dropwise to asolution of 1 (30 mmol) in H2O (100 mL), and after stirring atroom temperature for 2 h, the mixture was extracted withCH2Cl2, dried with anhydrous Na2SO4, and concentrated invacuo to afford hydrazine 3. Then, a 40% aqueous solution ofglyoxal (25 mL) was added to a solution of 3 (62 mmol) inH2O (100 mL). The mixture was heated at reflux for 5 h andextracted with ethyl acetate. The organic phase was dried,filtered, and concentrated to give 4 as yellow solid. Tetrahydropyrrole(8 mmol) was added to a solution of 4 (24 mmol)in MeOH (45 mL), and after stirring at room temperature for2 h, 1-(N-Boc)-4-piperidone (28 mmol) was subsequentlyadded, with the mixture heated at reflux for 24 h. Themixture was concentrated in vacuo and purified via columnchromatography to give 5. The Boc-protecting group wasremoved using trifluoroacetic acid in CH2Cl2 to provide 6.Finally, appropriate quinoline-4-carboxylic acid derivatives(1 mmol) was added to the mixture of 6 (1.1 mmol), HATU(1.2 mmol), and triethylamine (0.2 mL) in DMF (10 mL). Themixture was stirred at room temperature overnight, andthen added to ice water. The resulting precipitate wasfiltered, dried, and purified by column chromatography togive target compounds 7a~7m and 7q~7u.
References:
Huang, Tonghui;Wu, Xin;Yan, Shirong;Liu, Tianya;Yin, Xiaoxing [European Journal of Medicinal Chemistry,2021,vol. 212,art. no. 113036]