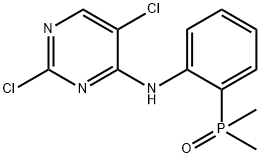
(2-((2,5-Dichloropyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide synthesis
- Product Name:(2-((2,5-Dichloropyrimidin-4-yl)amino)phenyl)dimethylphosphine oxide
- CAS Number:1197953-49-3
- Molecular formula:C12H12Cl2N3OP
- Molecular Weight:316.12
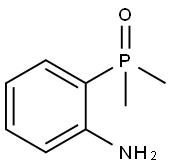
1197953-47-1
172 suppliers
inquiry
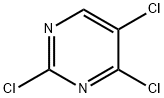
5750-76-5
358 suppliers
$5.00/1g

1197953-49-3
147 suppliers
inquiry
Yield:1197953-49-3 90.3%
Reaction Conditions:
with dipotassium hydrogenphosphate in N,N-dimethyl-formamide at 65; for 4 h;
Steps:
1 Example 1Synthesis of Intermediate 1 (2,5-dichloro-N-(2-(dimethylphosphine)phenyl)pyrimidine-4-amine).
Combine 2,4,5-trichloropyrimidine (AP-2) (15.2g, 83mmol), 2,4,5-trichloropyrimidine and 2-(dimethyloxyphosphoryl)aniline (AP-1) (9.4 g, 55.6 mmol, and K2HPO4 (15.3 g, 110 mmol) in a suspension (55 mL) in DMF, stirred at 65° C. for 4 hours. After cooling, the reaction mixture was filtered, the filter cake was washed with ethyl acetate (20 ml), and the filtrate was evaporated. Dissolve the residue in ethyl acetate (50ml), extract three times with saturated sodium chloride solution (3×100ml), combine the organic centers, spin to concentrate to a yellow-white solid, and beat three times with PE (3×20ml),The desired product 12.0 g AP-3 was obtained as a yellow-white solid. Yield: 90.3%, HPLC purity>98.0%.
References:
CN111138492,2020,A Location in patent:Paragraph 0048-0050
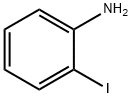
615-43-0
412 suppliers
$5.00/5g

1197953-49-3
147 suppliers
inquiry
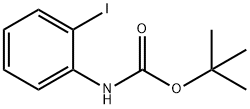
161117-84-6
30 suppliers
$119.00/1g

1197953-49-3
147 suppliers
inquiry