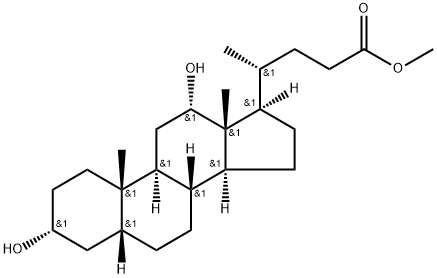
12β-Deoxycholic Acid Methyl Ester 3α-Benzoate synthesis
- Product Name:12β-Deoxycholic Acid Methyl Ester 3α-Benzoate
- CAS Number:5969-28-8
- Molecular formula:C32H46O5
- Molecular Weight:510.72

17225-41-1
4 suppliers
inquiry

5969-28-8
2 suppliers
inquiry
Yield:5969-28-8 85%
Reaction Conditions:
with lithium tri-t-butoxyaluminum hydride in tetrahydrofuran; for 24 h;Inert atmosphere;
Steps:
11 Synthesis of Compound 6
Under nitrogen protection,3000 ml of a three-necked round bottom flask was added compound 5 (170 g, 334 mmol)Tetrahydrofuran (1020 ml, 6 V),Stirring dissolved.Control system temperature at -5 ~ 5 ,A solution of 1 mol / L tri-t-butoxyaluminum hydride in tetrahydrofuran (501 mL, 501 mmol)After the dropwise addition,Stirring was continued at -5 to 5 ° C for 24 hours.HPLC monitoring of compound 5 (aera) <1%Stop the reaction.The system cooling,Keep the system temperature not higher than 10 ,2 mol / L hydrochloric acid (600 ml) was added dropwise,Dispensing,Combine organic phase,Saturated with sodium chloride (170 ml, 1 V) and 2 mol / L hydrochloric acid (170 ml, 1 V)The organic phase was obtained by liquid separation,Washed with saturated sodium chloride (340 ml, 2 V)The organic phase was obtained by liquid separation,The organic phase is depressurized to remove part of the solvent,Leaving about 300ml of solvent volume,Add n-heptane (1700 ml, 10V) with stirring,Dispensing,To obtain an organic phase,After the tetrahydrofuran was removed by distillation under reduced pressure (judged by the rate of the distilled liquid and n-heptane)While ensuring that the system volume of about 1700ml solution,The mixture was stirred at 10 to 25 ° C for 2 hours,filter,The filter cake was washed with a small amount of n-heptane,Drained,Compound 6 (145 g, yield 85%) was obtained.
References:
TW2016/41512,2016,A Location in patent:Paragraph 0059; 0060; 0061; 0062; 0063

98-88-4
578 suppliers
$10.00/5g

5969-28-8
2 suppliers
inquiry
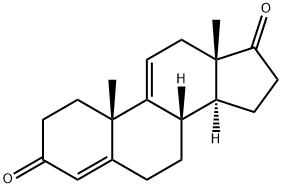
1035-69-4
35 suppliers
inquiry

5969-28-8
2 suppliers
inquiry
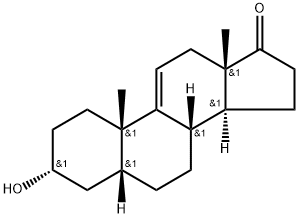
571-49-3
9 suppliers
inquiry

5969-28-8
2 suppliers
inquiry