
tert-butyl 4-(3-nitropyrazol-l-yl)piperidine-l-carboxylate synthesis
- Product Name:tert-butyl 4-(3-nitropyrazol-l-yl)piperidine-l-carboxylate
- CAS Number:1201935-68-3
- Molecular formula:C13H20N4O4
- Molecular Weight:296.32
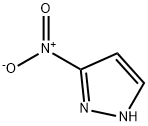
26621-44-3
211 suppliers
$6.00/5g

109384-19-2
469 suppliers
$5.00/1g

1201935-68-3
15 suppliers
$285.00/100mg
Yield:1201935-68-3 23%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran at 20; for 12 h;
Steps:
Synthesis of tert-butyl 4-(3-nitro-1H-pyrazol-1-yl)piperidine-1-carboxylate (12)
To a stirred solution of tert-butyl 4-hydroxypiperidine-1-carboxylate 10 (8.90 g, 44.21 mmol) and 3-nitro-1H-pyrazole 11 (5.00 g, 44.21 mmol) in THF (80 ml) was added TPP (17.3 g, 65.99 mmol) and followed by dropwise addition of DEAD (10.3 mL, 65.97 mmol) at room temperature.
The reaction mixture was further stirred at room temperature for 12 h.
After completion of the reaction (monitored by TLC), the reaction mixture was quenched with water (50 mL) and extracted with ethyl acetate (2*50 mL).
The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure.
The resulting crude compound was purified by silica gel column chromatography eluting with 0-30% ethyl acetate in n-hexane to afford 3.00 g (23% yield) of the title compound 12 as colorless oil.
Reaction was monitored by TLC(LC system: 50% ethyl acetate in n-hexane; Rf=0.4)
References:
US2021/70731,2021,A1 Location in patent:Paragraph 0157-0159

26621-44-3
211 suppliers
$6.00/5g
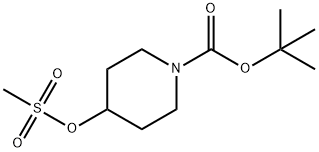
141699-59-4
233 suppliers
$6.00/1g

1201935-68-3
15 suppliers
$285.00/100mg

109384-19-2
469 suppliers
$5.00/1g

1201935-68-3
15 suppliers
$285.00/100mg