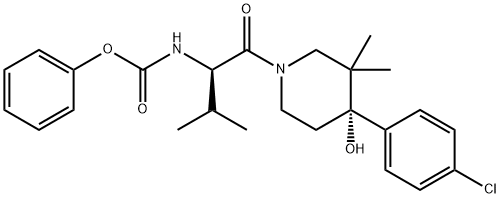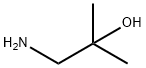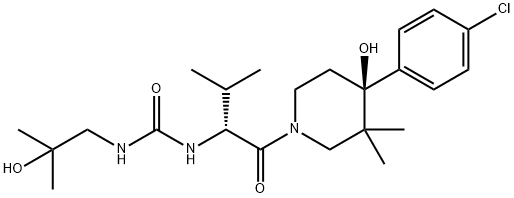
Urea, N-[(1R)-1-[[(4S)-4-(4-chlorophenyl)-4-hydroxy-3,3-diMethyl-1-piperidinyl]carbonyl]-2-Methylpropyl]-N'-(2-hydroxy-2-Methylpropyl)- synthesis
- Product Name:Urea, N-[(1R)-1-[[(4S)-4-(4-chlorophenyl)-4-hydroxy-3,3-diMethyl-1-piperidinyl]carbonyl]-2-Methylpropyl]-N'-(2-hydroxy-2-Methylpropyl)-
- CAS Number:1202400-18-7
- Molecular formula:C23H36ClN3O4
- Molecular Weight:454
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 20;Inert atmosphere;Reflux;
Steps:
1.8
Step 8: Compound of Formula I Under a nitrogen atmosphere, phenyl (R)-1-((S)-4-(4-chlorophenyl)-4-hydroxy-3,3-dimethylpiperidin-1-yl)-3-methyl-1-oxobutan-2-ylcarbamate (16.0 g, 34.9 mmol), 1-amino-2-methylpropan-2-ol (3.42 g, 38.3 mmol) and DIPEA (6.70 mL, 38.3 mmol) were mixed with stirring in MeCN (30 mL) at rt. The resulting suspension was heated to reflux, during which time the suspension became a colorless solution. After stirring at reflux for about 20 minutes, solids precipitated. After stirring at reflux for 1.5 h, 20 ml of acetonitrile and another 0.1 equiv. of 1-amino-2-methylpropan-2-ol and DIPEA were added. The reaction mixture was stirred for an additional 1.5 h. After this time, the reaction mixtured was removed from heating and allowed to cool to rt. While cooling to rt, water was added to precipitate the product (-240 mL) and the resulting free flowing suspension was stirred overnight. At the conclusion of this period, the resulting solids were collected by filtration, rinsed 2 times with water and then dried under high vacuum for 6 hours to give 15.4 grams of solids. These solids (and an additional 1 gram of pilot batch) were slurried in 50 mL of acetone at rt with stirring, and then 3 times the volume of water (150 mL) was added. The free-flowing suspension was stirred overnight. After this time, the resulting solids were collected by filtration, rinsed twice with water and then dried for 48 h to give 15.2 grams of the Compound of Formula I as a solid. 1H NMR (500 MHz, methanol-d4, rotameric) δ ppm 7.47 (dd, J=15.4, 8.8 Hz, 4 H), 7.31 (dd, J=8.5, 5.2 Hz, 4 H), 4.71 (dd, J=12.1, 6.1 Hz, 2 H), 4.54 (ddd, J=12.9, 2.5, 2.2 Hz, 1 H), 3.98-4.08 (m, 2 H), 3.58-3.68 (m, 2 H), 3.48 (dd, J=12.9, 1.4 Hz, 1 H), 3.13-3.21 (m, 2 H), 3.06-3.14 (m, 4 H), 2.70 (td, J=13.6, 4.7 Hz, 1 H), 2.61 (td, J=13.5, 5.0 Hz, 1 H), 2.09 (dq, J=13.2, 6.6 Hz, 1 H), 1.95 (dq, J=13.3, 6.7 Hz, 1 H), 1.60 (ddd, J=13.9, 2.5, 2.3 Hz, 1 H), 1.51 (ddd, J=14.2, 2.6, 2.5 Hz, 1 H), 1.16 (s, 6 H), 1.14 (d, J=1.7 Hz, 6 H), 1.05 (d, J=7.2 Hz, 3 H), 0.98 (d, J=7.2 Hz, 3 H), 0.94 (d, J=6.6 Hz, 3 H), 0.91 (d, J=6.6 Hz, 3 H), 0.82 (s, 3 H), 0.81 (s, 3 H), 0.79 (s, 3 H), 0.75 (s, 3 H). 13C NMR (126 MHz, methanol-d4) δ ppm 173.6, 173.3, 161.1, 160.8, 144.8, 144.6, 133.82 (2 C, s), 130.2 (4 C, s), 128.3 (4 C, s), 76.0, 76.0, 71.7, 71.7, 55.9, 55.2, 55.1, 51.8 (2 C, s), 51.1, 43.0, 40.4, 39.9, 39.3, 34.8, 33.7, 33.1, 32.4, 27.2 (2 C, s), 27.1 (2 C, s), 23.1, 22.8, 21.4, 21.1, 20.3, 19.8, 17.9, 17.7, m/z: 454.2 [M+]+.
References:
US2011/82113,2011,A1 Location in patent:Page/Page column 23-24
690660-22-1
2 suppliers
inquiry
![Urea, N-[(1R)-1-[[(4S)-4-(4-chlorophenyl)-4-hydroxy-3,3-diMethyl-1-piperidinyl]carbonyl]-2-Methylpropyl]-N'-(2-hydroxy-2-Methylpropyl)-](/CAS/20150408/GIF/1202400-18-7.gif)
1202400-18-7
2 suppliers
inquiry
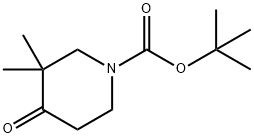
324769-06-4
145 suppliers
$9.00/250mg
![Urea, N-[(1R)-1-[[(4S)-4-(4-chlorophenyl)-4-hydroxy-3,3-diMethyl-1-piperidinyl]carbonyl]-2-Methylpropyl]-N'-(2-hydroxy-2-Methylpropyl)-](/CAS/20150408/GIF/1202400-18-7.gif)
1202400-18-7
2 suppliers
inquiry
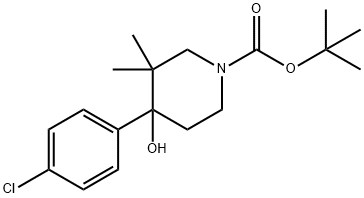
475085-56-4
2 suppliers
inquiry
![Urea, N-[(1R)-1-[[(4S)-4-(4-chlorophenyl)-4-hydroxy-3,3-diMethyl-1-piperidinyl]carbonyl]-2-Methylpropyl]-N'-(2-hydroxy-2-Methylpropyl)-](/CAS/20150408/GIF/1202400-18-7.gif)
1202400-18-7
2 suppliers
inquiry
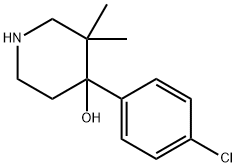
690660-21-0
5 suppliers
inquiry
![Urea, N-[(1R)-1-[[(4S)-4-(4-chlorophenyl)-4-hydroxy-3,3-diMethyl-1-piperidinyl]carbonyl]-2-Methylpropyl]-N'-(2-hydroxy-2-Methylpropyl)-](/CAS/20150408/GIF/1202400-18-7.gif)
1202400-18-7
2 suppliers
inquiry