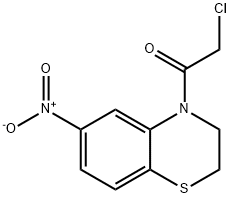
4-(2-Chloroacetyl)-6-nitro-2H-1,4-benzothiazine, 97% synthesis
- Product Name:4-(2-Chloroacetyl)-6-nitro-2H-1,4-benzothiazine, 97%
- CAS Number:1202773-87-2
- Molecular formula:C10H9ClN2O3S
- Molecular Weight:272.71

1193387-98-2
9 suppliers
$463.00/1g

79-04-9
352 suppliers
$12.00/5g

1202773-87-2
9 suppliers
inquiry
Yield:1202773-87-2 100%
Reaction Conditions:
in tetrahydrofuran at 60; for 0.166667 h;
Steps:
24
2-Chloro-1-(6-nitro-2H-benzo[b][1,4]thiazin-4(3H)-yl)ethanone: To a stirred solution of 6-nitro-3,4-dihydro-2H-benzo[b][1,4]thiazine (620 mg, 3.16 mmol) in THF (10 mL) was added 2-chloroacetyl chloride (0.277 mL, 3.48 mmol). The resulting mixture was then stirred at 60° C. for 10 minutes The mixture was then diluted with ethyl acetate and washed with water (3×), 1:1 water:saturated sodium carbonate, and brine. The organic phase was dried, filtered, and concentrated, giving the desired product (860 mg, 100%). 1H NMR (DMSO-d6) δ 8.36 (d, J=2.1 Hz, 1H), 7.96 (dd, J=8.7, 2.1 Hz, 1H), 7.52 (d, J=8.7 Hz, 1H), 4.63 (s, 2H), 3.99-3.92 (m, 2H), 3.39-3.33 (m, 2H); ESI-MS (m/z, %): 295 (M+Na, 68), 273 (MH+, 100), 197 (43).
References:
US2010/9975,2010,A1 Location in patent:Page/Page column 61