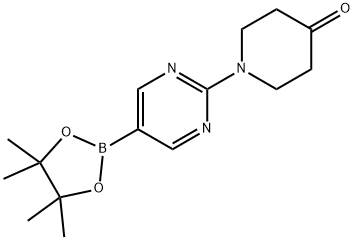
1-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-yl)piperidin-4-one synthesis
- Product Name:1-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-yl)piperidin-4-one
- CAS Number:1202805-27-3
- Molecular formula:C15H22BN3O3
- Molecular Weight:303.16
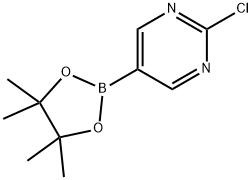
1003845-08-6
110 suppliers
$12.00/250mg
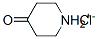
41979-39-9
117 suppliers
$15.00/10mg

1202805-27-3
13 suppliers
inquiry
Yield:1202805-27-3 50.24%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 100; for 12 h;
Steps:
a) l-[5-(4,4, 5, 5-Tetramethyl-l, 3, 2-dioxaborolan-2-yl)pyrimidin-2-yl ] piper idin-4-one
To a solution of piperidin-4-one;hydrochloride (2.79 g, 20.58 mmol, 1.1 eq) in DMF (50 mL) was added 2-chloro-5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)pyrimidine (4.5 g, 18.71 mmol, 1 eq) and potassium carbonate (5.43 g, 39.29 mmol, 2.1 eq). Then the reaction was stirred at 100 °C for 12 h. Aqueous sodium chloride solution (300.0 ml) was added to quench the reaction, the reaction mixture was extracted with three 50-ml portions of ethyl acetate. The combined extracts were concentrated under reduced pressure to give a residue. The residue was purified by flash chromatography to give l-[5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)pyrimidin-2- yl]piperidin-4-one (3 g, 9.9 mmol, 50.24% yield) as a white solid. MS m/e: 222.2 ([M+H+J+).
References:
WO2021/255212,2021,A1 Location in patent:Page/Page column 602

79099-07-3
543 suppliers
$5.00/5g

1202805-27-3
13 suppliers
inquiry