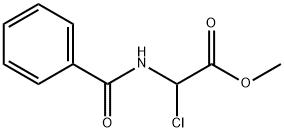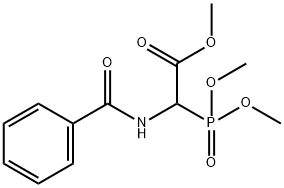
Acetic acid, 2-(benzoylamino)-2-(dimethoxyphosphinyl)-, methyl ester synthesis
- Product Name:Acetic acid, 2-(benzoylamino)-2-(dimethoxyphosphinyl)-, methyl ester
- CAS Number:120341-28-8
- Molecular formula:C12H16NO6P
- Molecular Weight:301.23
Yield: 59%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 17 h;
Steps:
65 Example 65; BENZOYLAMINO- (DINAETHOXY-PHOSPHOY ; YL)-ACETIC ACID METHYL ESTER.
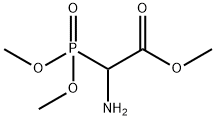
A dry 100 ml round-bottomed flask with an inert nitrogen atmosphere was charged with benzoyl chloride (1.26 ml, 10.87 mmol), anhydrous dichloromethane (10 ml), and triethylamine (2.17 ml, 16.3 mmol). The solution was cooled to 0 C in a brine ice bath and a solution containing amino- (dimethoxy-phosphoryl)-acetic acid methyl ester (9.06 mmol) in anhydrous dichloromethane (10 ml) was added. The reaction was stirred for one hour at 0 C, and then at room temperature for sixteen hours. The reaction was diluted with ethyl acetate (100 ml), washed with water, and brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The product was purified on a silica gel flash chromatography column which was eluted with a gradient of 97: 3 to 95: 5 dichloromethane: methanol. The fractions containing the product were combined and concentrated. The product was obtained as a solid, 1.61 g, 59% yield, and had a melting point =112-114 C.
References:
TRIANGLE PHARMACEUTICALS, INC. WO2004/50613, 2004, A2 Location in patent:Page 87
![methyl ([(benzyloxy)carbonyl]amino)-(dimethoxyphosphoryl)acetate](/CAS/20180601/GIF/100945-15-1.gif)
100945-15-1
3 suppliers
inquiry

120341-28-8
3 suppliers
inquiry