
2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)- synthesis
- Product Name:2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-
- CAS Number:1204185-88-5
- Molecular formula:C18H21F3O5
- Molecular Weight:374.35
![(+)-(3AR,4R,5R,6AS)-HEXAHYDRO-5-HYDROXY-4-[(1E,3R)-3-HYDROXY-4-(3-TRIFLUOROMETHYL)PHENOXY-1-BUTENYL]-2H-CYCLOPENTA[B]FURAN-2-ONE](/CAS/GIF/53872-60-9.gif)
53872-60-9
114 suppliers
$94.00/50mg
![2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-](/CAS/20180703/GIF/1204185-88-5.gif)
1204185-88-5
18 suppliers
inquiry
Yield:1204185-88-5 74.63%
Reaction Conditions:
with diisobutylaluminium hydride in tetrahydrofuran at -60; for 4 h;
Steps:
2.6 Step 6: Preparation of a compound of formula V {(3aR, 4R, 5R, 6aS) -4 - ((R, E) -3- hydroxy- 4- (3- (trifluoromethyl) phenoxy) Anil) hexahydro-2H-cyclopenta [b] furan-2,5-diol}
The compound of formula IV (2 g, 5.371 mmol) prepared in step 5 was dissolved in 20 mL of THF and the reaction was cooled to -60 ° C and DIBAL-H (13.5 mL, 13.428 mmol, 2.5 eq) And the mixture was stirred at -60 ° C for 4 hours. 50 mL of a 10% potassium sodium tartrate solution was added dropwise at -60 DEG C, and 50 mL of ethyl acetate was added at 28 DEG C, followed by stirring for 2 hours to separate the layers. The organic layer was washed with 100 mL of water, dried over MgSO, and concentrated under reduced pressure. The colorless oil was columned with methylene chloride: MeOH = 9: 1 to give a colorless oil (1.5 g, 74.63%).
References:
KR2017/25682,2017,A Location in patent:Paragraph 0136; 0162-0164
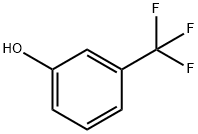
98-17-9
440 suppliers
$5.00/10g
![2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-](/CAS/20180703/GIF/1204185-88-5.gif)
1204185-88-5
18 suppliers
inquiry

588-26-1
21 suppliers
inquiry
![2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-](/CAS/20180703/GIF/1204185-88-5.gif)
1204185-88-5
18 suppliers
inquiry
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate](/CAS/GIF/54094-19-8.gif)
54094-19-8
132 suppliers
$65.00/1g
![2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-](/CAS/20180703/GIF/1204185-88-5.gif)
1204185-88-5
18 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/54142-64-2.gif)
54142-64-2
54 suppliers
inquiry
![2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-, (3aR,4R,5R,6aS)-](/CAS/20180703/GIF/1204185-88-5.gif)
1204185-88-5
18 suppliers
inquiry