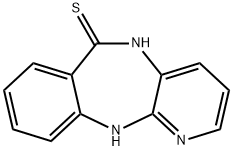
5,11-Dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-thione synthesis
- Product Name:5,11-Dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-thione
- CAS Number:1204296-84-3
- Molecular formula:C12H9N3S
- Molecular Weight:227.29
![5,11-Dihydropyrido[2,3-b][1,4]benzodiazepin-6-one](/CAS/GIF/885-70-1.gif)
885-70-1
82 suppliers
$30.00/1g
![5,11-Dihydro-6H-pyrido[2,3-b][1,4]benzodiazepine-6-thione](/CAS/GIF/1204296-84-3.gif)
1204296-84-3
4 suppliers
inquiry
Yield:1204296-84-3 74%
Reaction Conditions:
with Lawessons reagent at 150; for 0.5 h;
Steps:
5.1.1
Example 1Synthesis of 6-(methylthio)-11H-benzo[e]pyrido[3,2-b][1,4]diazepineStep 1. Synthesis of 5H-benzo[e]pyrido[3,2-b][1,4]diazepine-6(11H)-thioneTo a mixture of 5H-benzo[e]pyrido[3,2-b][1,4]diazepin-6(11H)-one (0.2 g, 0.948 mmol) in Dowtherm A (3 mL) was added Laweson's reagent (0.384 g, 0.948 mmol). The reaction mixture was heated at 150° C. for 30 minutes. Once the reaction was complete the reaction mixture was allowed to cool to room temperature and a 1:2 mixture of EtOAc and hexanes (10 ml) was added. The solid was filtered, washed with a 1:2 mixture of EtOAc and hexanes (10 ml) and dried under high vacuum. The desired product, 5H-benzo[e]pyrido[3,2-b][1,4]diazepine-6(11H)-thione was obtained as a reddish brown solid (0.16 g, 74%). M.p.=178-180° C.; 400 MHz 1H NMR (DMSO-d6) δ: 11.99 (s, 1H), 10.4-9.6 (br s, 1H), 8.40 (s, 1H), 8.0-7.8 (m, 2H), 7.47-7.3 (m, 2H), 7.08-6.9 (m, 2H); LCMS [M+H]: 228.
References:
US2011/245236,2011,A1 Location in patent:Page/Page column 93