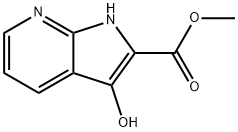
1H-Pyrrolo[2,3-b]pyridine-2-carboxylic acid, 3-hydroxy-, Methyl ester synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine-2-carboxylic acid, 3-hydroxy-, Methyl ester
- CAS Number:1204475-92-2
- Molecular formula:C9H8N2O3
- Molecular Weight:192.17
Yield:-
Reaction Conditions:
in methanol;
Steps:
17
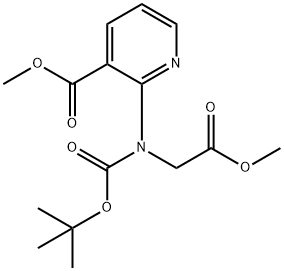
Example 17Synthesis of 3 -hydroxy- lH-pyrrolo [ 2 , 3 -£>] pyridine- 2 -carboxylic acid32Protection of 2-aminonicotinic acid (25) as its N-hoc derivative proceeds under standard conditions gives 2- ( tert-butoxycarbonylamino) nicotinic acid (26) . Reaction of 26 with aqueous formaldehyde in the presence of 4-dimethylaminopyridine (DMAP) provides tert-butyl 4-oxo-2 , 4-dihydro-lH-pyrido [2 , 3-d] [1,3] oxazine-1-carboxylate(27) that is treated with aqueous sodium cyanide in tetrahydrofuran (THF) to afford 2- ( tert-butoxycarbonyl(cyanomethyl) amino) nicotinic acid (28) . .Hydrolysis of 28 with aqueous sodium hydroxide followed by acidification and reintroduction of the Boc group gives2- ( tert-butoxycarbonyl (carboxymethyl) amino) nicotinic acid(29) . Esterification of 29 with diazomethane gives methyl 2 - ( tert-butoxycarbonyl ( 2 -methoxy-2 -oxoethyl ) amino ) nicotinate (30) that is subjected to intramolecular Claisen condensation to give methyl3 -hydroxy-lH-pyrrolo [2 , 3-2?] pyridine-2-carboxylate (31) . Careful saponification of 31 gives 3-hydroxy-lH-pyrrolo[2 , 3-jb]pyridine-2-carboxylic acid (32).
References:
WO2010/5528,2010,A2 Location in patent:Page/Page column 95-96