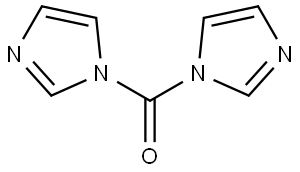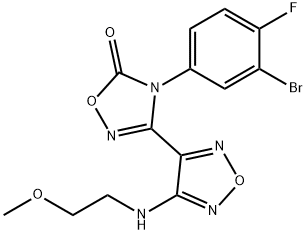
1,2,4-Oxadiazol-5(4H)-one, 4-(3-bromo-4-fluorophenyl)-3-[4-[(2-methoxyethyl)amino]-1,2,5-oxadiazol-3-yl]- synthesis
- Product Name:1,2,4-Oxadiazol-5(4H)-one, 4-(3-bromo-4-fluorophenyl)-3-[4-[(2-methoxyethyl)amino]-1,2,5-oxadiazol-3-yl]-
- CAS Number:1204669-63-5
- Molecular formula:C13H11BrFN5O4
- Molecular Weight:400.16
Yield:1204669-63-5 98%
Reaction Conditions:
in ethyl acetate;
Steps:
1.G Step G:
Step G: 4-(3-Bromo-4-fluorophenyl)-3-{4-[(2-methoxyethyl)amino]-1,2,5-oxadiazol-3-yl}-1,2,4-oxadiazol-5(4H)-one A mixture of N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-[(2-methoxyethyl)amino]-1,2,5-oxadiazole-3-carboximidamide (76.5 g, 0.204 mol), 1,1'-carbonyldiimidazole (49.7 g, 0.307 mol), and ethyl acetate (720 mL) was heated to 60° C. and stirred for 20 min. LCMS indicated reaction completed. The reaction was cooled to room temperature, washed with 1 N HCl (2*750 mL), dried over sodium sulfate, and concentrated to give the desired product (80.4 g, 98%) as a crude brown solid. LCMS for C13H12BrFN5O4 (M+H)+: m/z=400.0, 402.0. 1H NMR (400 MHz, DMSO-d6): δ 7.94 (t, J=8.2 Hz, 1H), 7.72 (dd, J=9.1, 2.3 Hz, 1H), 7.42 (m, 1H), 6.42 (t, J=5.7 Hz, 1H), 3.46 (t, J=5.4 Hz, 2H), 3.36 (t, J=5.8 Hz, 2H), 3.26 (s, 3H).
References:
US2010/15178,2010,A1
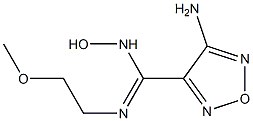
1204669-59-9
0 suppliers
inquiry
![1,2,4-Oxadiazol-5(4H)-one, 4-(3-bromo-4-fluorophenyl)-3-[4-[(2-methoxyethyl)amino]-1,2,5-oxadiazol-3-yl]-](/CAS/20180703/GIF/1204669-63-5.gif)
1204669-63-5
6 suppliers
inquiry
![1,2,5-Oxadiazole-3-carboximidamide,N-hydroxy-4-[(2-methoxyethyl)amino]-](/CAS/20180702/GIF/1204669-60-2.gif)
1204669-60-2
0 suppliers
inquiry
![1,2,4-Oxadiazol-5(4H)-one, 4-(3-bromo-4-fluorophenyl)-3-[4-[(2-methoxyethyl)amino]-1,2,5-oxadiazol-3-yl]-](/CAS/20180703/GIF/1204669-63-5.gif)
1204669-63-5
6 suppliers
inquiry
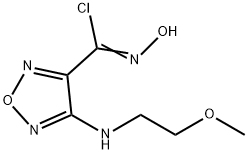
1204669-61-3
12 suppliers
$45.00/2.5mg
![1,2,4-Oxadiazol-5(4H)-one, 4-(3-bromo-4-fluorophenyl)-3-[4-[(2-methoxyethyl)amino]-1,2,5-oxadiazol-3-yl]-](/CAS/20180703/GIF/1204669-63-5.gif)
1204669-63-5
6 suppliers
inquiry
![1,2,5-Oxadiazole-3-carboximidamide,N'-(3-bromo-4-fluorophenyl)-N-hydroxy-4-[(2-methoxyethyl)amino]-](/CAS/20180703/GIF/1204669-62-4.gif)