
4-[5-(TRIFLUOROMETHYL)-1,2,4-OXADIAZOL-3-YL]-1,2,5-OXADIAZOL-3-AMINE synthesis
- Product Name:4-[5-(TRIFLUOROMETHYL)-1,2,4-OXADIAZOL-3-YL]-1,2,5-OXADIAZOL-3-AMINE
- CAS Number:120493-19-8
- Molecular formula:C5H2F3N5O2
- Molecular Weight:221.1
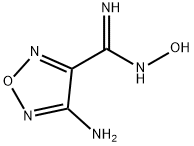
13490-32-9
114 suppliers
$12.00/1mg
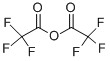
407-25-0
439 suppliers
$9.00/1g
![4-[5-(TRIFLUOROMETHYL)-1,2,4-OXADIAZOL-3-YL]-1,2,5-OXADIAZOL-3-AMINE](/StructureFile/ChemBookStructure2/GIF/CB7119680.gif)
120493-19-8
8 suppliers
$569.00/5g
Yield:120493-19-8 72%
Reaction Conditions:
Stage #1: 4-amino-N-hydroxy-furazan-3-carboxamidine;trifluoroacetic anhydride in toluene; for 3 h;Reflux;
Stage #2: in methanol;water; for 1 h;Reflux;
Steps:
Synthesis of 4-(5-trifluoromethyl-1,2,4-oxadiazol-3-yl)furazan-3-amine (6a) and its reaction with hydrazine. Amidoxime of 4-aminofurazan-3-carboxylic acid (4)(2.0 g, 0.014 mol) was gradually added to stirred toluene (25 ml), then (CF3CO)2O (8.8 g, 0.042 mol) was added andthe mixture was refluxed for 3 h. Solvent was removedin vacuo, the residue was dissolved in MeOH (10 ml), treated with H2O (10 ml), and refluxed for 1 h. The mixture was cooled to room temperature, the precipitate of compound 6 was filtered off. Yield 2.23 g (72%), colorless plates, mp 126-127°C (MeOH) (mp 126-128°C (2-PrOH)40). IR spectrum, ν, cm-1: 3469, 3339, 1628, 1612,1554, 1463, 1335, 1216, 1177, 997, 967, 930, 775, 762,575, 530.
References:
Stepanov, Andrei I.;Sannikov, Vladimir S.;Dashko, Dmitry V.;Roslyakov, Alexey G.;Astrat'Ev, Alexander A.;Stepanova, Elena V. [Chemistry of Heterocyclic Compounds,2015,vol. 51,# 4,p. 350 - 360][Khim. Geterotsikl. Soedin.,2015,vol. 51,# 4,p. 350 - 360,11]