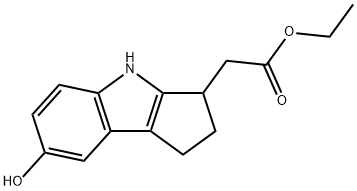
ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02032) synthesis
- Product Name:ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02032)
- CAS Number:1206124-13-1
- Molecular formula:C15H17NO3
- Molecular Weight:259.3
Yield:1206124-13-1 88.9%
Reaction Conditions:
Stage #1: 2-(7-methoxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acidwith boron tribromide in dichloromethane at -5 - 0; for 1 h;
Stage #2: ethanol in dichloromethane at 0 - 40; for 0.5 h;
Steps:
Telescoped procedure for the preparation of indole derivative 16
To a solution of BBr3 (115 g, 43.3 mL, 458 mmol, 3 equiv) in CH2Cl2 (70 mL), a suspension of 2-(7-methoxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid (14a,37.44 g, 153 mmol) in CH2Cl2 (300 mL) was added slowly while maintaining the reaction temperature between -5 to 0 °C. The resulting dark brownsuspension was stirred at -5 to °0 C for an additional 1 h. EtOH (187 mL) was added dropwise to the reaction mixture while maintaining the temperature between 0 and 10 °C. The resulting solution was heated at 40 °C for 30 min. The solution was cooled and the pH adjusted to 8 by adding 10 N NaOH (142.9 mL,1.43 mol) slowly while maintaining the temperature between 0 and 3 °C. The solvent was removed under reduced pressure until about 200 mL of concentrate remained. The pH was adjusted to about 7 with concentrated HCl, the suspension filtered, the solids washed with H2O (3 200 mL) anddried under vacuum at ambient temperature overnight. The light brown material was dissolved in EtOAc (200 mL) and filtered washing the solids with EtOAc. The combined organics were washed with saturated aqueous NaHCO3.(2 200 mL), brine (200 mL), dried (Na2SO4) and the solvent rotaryevaporated to afford ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate (35.2 g, 88.9%) as a light brown solid. 1H NMR (400 MHz, DMSOd6)d ppm 10.25 (s, 1H), 8.47 (s, 1H), 7.07 (d, J = 8.6 Hz, 1H), 6.62 (d, J = 2.1 Hz,1H), 6.49 (dd, J = 8.6, 2.3 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.49-3.42 (m, 1H),2.76 (dd, J = 15.7, 5.5 Hz, 1H), 2.71-2.55 (m, 3H), 2.42 (dd, J = 15.7, 8.9 Hz, 1H),2.11-2.03 (m, 1H), 1.20 (t, J = 7.1 Hz, 3H). LCMS m/z = 260.1 [M+H]+.
References:
Montalban, Antonio Garrido;Ma, You-An;Johannsen, Steve;Tandel, Sagun;Martinelli, Michael J. [Tetrahedron Letters,2015,vol. 56,# 2,p. 378 - 381]
![Acetic acid, 2-[1,4-dihydro-7-(phenylmethoxy)cyclopent[b]indol-3(2H)-ylidene]-, ethyl ester](/CAS/20210305/GIF/1318262-62-2.gif)
1318262-62-2
2 suppliers
inquiry
![ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02032)](/CAS/20180703/GIF/1206124-13-1.gif)
1206124-13-1
35 suppliers
inquiry
![Cyclopent[b]indole-3-acetic acid, 1,2,3,4-tetrahydro-7-methoxy-](/CAS/20210305/GIF/1206125-25-8.gif)
1206125-25-8
1 suppliers
inquiry
![ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02032)](/CAS/20180703/GIF/1206124-13-1.gif)
1206124-13-1
35 suppliers
inquiry
![ethyl 2-(7-methoxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02031)](/CAS/20180703/GIF/1206124-14-2.gif)
1206124-14-2
3 suppliers
inquiry
![ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02032)](/CAS/20180703/GIF/1206124-13-1.gif)
1206124-13-1
35 suppliers
inquiry
20826-94-2
167 suppliers
$26.00/1g
![ethyl 2-(7-hydroxy-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate(WXG02032)](/CAS/20180703/GIF/1206124-13-1.gif)
1206124-13-1
35 suppliers
inquiry
