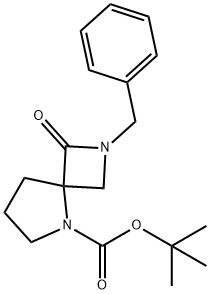
tert-butyl 2-benzyl-1-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate synthesis
- Product Name:tert-butyl 2-benzyl-1-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate
- CAS Number:1206970-01-5
- Molecular formula:C18H24N2O3
- Molecular Weight:316.39
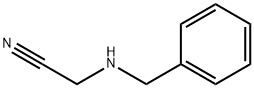
3010-05-7
34 suppliers
inquiry

145681-01-2
72 suppliers
$25.00/1g
![tert-butyl 2-benzyl-1-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate](/CAS/20150408/GIF/1206970-01-5.gif)
1206970-01-5
17 suppliers
$295.00/250mg
Yield:1206970-01-5 76%
Reaction Conditions:
Stage #1: (+/-)-N-tert-butoxycarbonylproline methyl esterwith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -70; for 1 h;Inert atmosphere;
Stage #2: benzylaminoacetonitrile in tetrahydrofuran;hexane at -70 - 20; for 13 h;Inert atmosphere;
Steps:
52 Intermediate 52: tert-Butyl 2-benzyl-3-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate
n-Butyllithum (268.7 mL, 0.672 mmol, 2.5 M in hexane) was added to a solution of diisopropylamine (70 g, 691.7 mmol) at -70°C in dry THF (1.5 L) under N2 then the mixture was stirred for1h at -70°C. A solution of 1-tert-butyl 2-methyl pyrrolidine-1,2-dicarboxylate (Intermediate 53, 140 g, 610 mmol) in anhydrous THF (360 mL) was then added dropwise at -70°C. After stirring at -70°C for1h, a solution of 2-(benzylamino)-acetonitrile (Intermediate 54, 45.9 g, 305.7 mmol) in anhydrous THF (360 mL) was added dropwise at -70°C over a period of1h. Then the resulting mixture was warmed to r.t. then stirred for 12h. Sat. NH4Cl (1.5 L) was then added and the resulting phases were separated. The aqueous solution was extracted with EtOAc (3 x 1 L). The combined organic solutions were washed with brine (3 x 1 L), dried (MgSO4) and concentrated in vacuo. Purification by FCC, eluting with 2:1 petroleum ether/EtOAc gave the title compound (75.5 g, 76%) as yellow oil; 1H NMR: (CDCl3) 1.44 (9H, m), 1.77 (1H, m), 1.93 (1H, m), 2.04 (1H, m), 2.37 (1H, m), 3.00 (1H, m), 3.40-3.67 (3H, m), 3.95 (1H, m), 4.25-4.89 (1H, m), 7.21-7.36 (5H, m).
References:
WO2013/14448,2013,A1 Location in patent:Page/Page column 103
15761-39-4
603 suppliers
$5.00/10g
![tert-butyl 2-benzyl-1-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate](/CAS/20150408/GIF/1206970-01-5.gif)
1206970-01-5
17 suppliers
$295.00/250mg

100-46-9
461 suppliers
$5.00/5G
![tert-butyl 2-benzyl-1-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate](/CAS/20150408/GIF/1206970-01-5.gif)
1206970-01-5
17 suppliers
$295.00/250mg
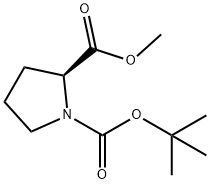
59936-29-7
224 suppliers
$6.00/5g
![tert-butyl 2-benzyl-1-oxo-2,5-diazaspiro[3.4]octane-5-carboxylate](/CAS/20150408/GIF/1206970-01-5.gif)
1206970-01-5
17 suppliers
$295.00/250mg