
3-AMino-2-benzyloxy-5-fluoro-6-Methyl-benzoic acid phenyl ester synthesis
- Product Name:3-AMino-2-benzyloxy-5-fluoro-6-Methyl-benzoic acid phenyl ester
- CAS Number:1207284-89-6
- Molecular formula:C21H18FNO3
- Molecular Weight:351.37
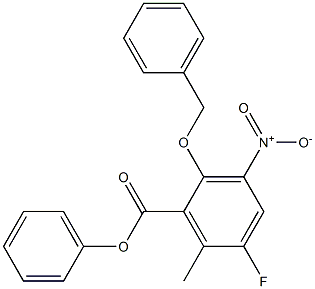
1207284-88-5
1 suppliers
inquiry

1207284-89-6
9 suppliers
inquiry
Yield:1207284-89-6 15.83 g
Reaction Conditions:
with sodium dithionite;water in tetrahydrofuran at 10 - 20.4;
Steps:
10
To a 1 L round bottom flask was added compound S10-2 (21.08 g, 55.40 mmol, 1.0 equiv) and THF (230 mL). The solution was cooled in a cold water bath to 10° C. To another 500 mL round bottom flask containing water (230 mL), sodium hydrosulfite (Na2S2O4, 56.7 g, 276.80 mmol, 5.0 equiv) was added slowly with stirring. The aqueous solution of sodium hydrosulfite was added to the THF solution of compound S10-2. The temperature quickly rose from 10° C. to 20.4° C. after the addition. The yellow suspension was stirred while the cold water bath slowly warmed up to room temperature overnight to give an orange cloudy solution. The reaction temperature during this period was between 15° C. to 19° C. TLC (heptane/EtOAc=9/1) showed the reaction was complete. The orange cloudy solution was diluted with EtOAc (460 mL). The organic layer was washed with water (150 mL×2) and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the crude product as a brown oil. The crude product was purified by flash silica gel column eluted with heptane/EtOAc 9/1 to yield the desired product S10-3 (15.83 g, 80%, 3 steps).
References:
US9315451,2016,B2 Location in patent:Page/Page column 153; 154; 155; 156
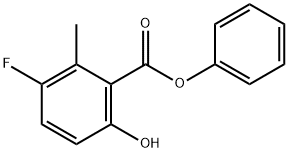
1207283-55-3
3 suppliers
inquiry

1207284-89-6
9 suppliers
inquiry
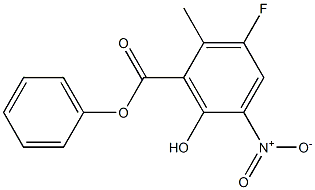
1207284-87-4
2 suppliers
inquiry

1207284-89-6
9 suppliers
inquiry
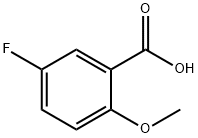
394-04-7
187 suppliers
$5.00/250mg

1207284-89-6
9 suppliers
inquiry

220901-72-4
15 suppliers
inquiry

1207284-89-6
9 suppliers
inquiry