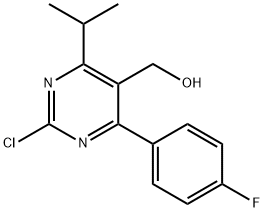
(2-Chloro-4-(4-fluorophenyl)-6-isopropylpyrimidin-5-yl)methanol synthesis
- Product Name:(2-Chloro-4-(4-fluorophenyl)-6-isopropylpyrimidin-5-yl)methanol
- CAS Number:1207460-23-8
- Molecular formula:C14H14ClFN2O
- Molecular Weight:280.73

488798-38-5
13 suppliers
inquiry

1207460-23-8
3 suppliers
inquiry
Yield:1207460-23-8 85%
Reaction Conditions:
with diisobutylaluminium hydride in toluene at 0; for 3 h;
Steps:
Synthetic procedure for the (2-Chloro-4-(4-fluorophenyl)-6-isopropylpyrimidin-5-yl)methanol (7).
To a solution of 6 (30 mmol) in toluene (100 mL) at 0 °C was added drop wise a 20%solution of diisobutylaluminium hydride (DIBAL) in toluene (85 mL). The reaction mixturewas further stirred for 2-3 h at 0 °C. The progress of reaction was monitored by thin layerchromatography. After completion, the reaction mixture was quenched with saturated icecold hydrochloric acid. The toluene layer was washed with water and brine. The organic layerwas separated, dried over anhydrous sodium sulfate and filtered. The filtrate was dried undervacuum. The solid product was purified by stirring in hexane or diisopropylether (DIPE) togive analytically pure product 7 with 85% yield.
References:
Patel, Anilkumar S.;Gandhi, Sahaj A.;Modh, Rajesh D.;Patel, Urmila H.;Naliapara, Yogesh T.;Kapuriya, Naval P. [Letters in Organic Chemistry,2021,vol. 18,# 8,p. 634 - 639] Location in patent:supporting information

459-57-4
597 suppliers
$6.00/25g

1207460-23-8
3 suppliers
inquiry

42558-54-3
386 suppliers
$10.00/5g

1207460-23-8
3 suppliers
inquiry