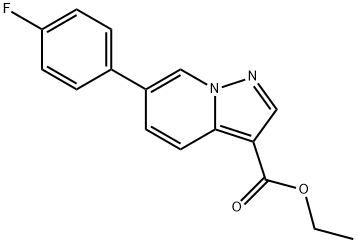
Pyrazolo[1,5-a]pyridine-3-carboxylic acid, 6-(4-fluorophenyl)-, ethyl ester synthesis
- Product Name:Pyrazolo[1,5-a]pyridine-3-carboxylic acid, 6-(4-fluorophenyl)-, ethyl ester
- CAS Number:1207557-16-1
- Molecular formula:C16H13FN2O2
- Molecular Weight:284.29
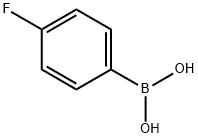
1765-93-1
550 suppliers
$9.00/1g
![Ethyl 6-broMopyrazolo[1,5-a]pyridine-3-carboxylate](/CAS/20150408/GIF/55899-30-4.gif)
55899-30-4
49 suppliers
$157.00/1/ G
![Pyrazolo[1,5-a]pyridine-3-carboxylic acid, 6-(4-fluorophenyl)-, ethyl ester](/CAS/20210111/GIF/1207557-16-1.gif)
1207557-16-1
0 suppliers
inquiry
Yield:1207557-16-1 77%
Reaction Conditions:
with sodium carbonate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water at 100; for 1.5 h;Suzuki Coupling;
Steps:
1.B
Step B: Suzuki CouplingA solution of ethyl 6-bromopyrazolo[l ,5-α] pyridine-3-carboxylate (4, 890 mg, 3.31 mmol) and p-fIuorophenylboronic acid (555 mg, 3.97 mmol) in dioxane (13.23 mL) and aq. sodium carbonate (2M, 4.5 mL, 9.00 mmol) deoxygenated. Pd(PPh3)* (382 mg, 0.3 mmol) was added to the reaction and system was heated by to 1000C and stirred for 1.5 hours. After cooling the reaction to room temperature, the reaction was filtered through a pad of silica and partitioned with DCM (10 rnL) and washed with water (2 x 15 mL). The organic was dried and concentrated, then dissolved in EtOAc (10 mL). The resulting gold precipitate was filtered off to yield 535 mg of the coupled product 5. The mother liquor was concentrated and additional product (185 mg) was recovered by column chromatography (10% acetone in hexanes) for a combined yield of 77% (720 mg, 2.53 mmol). MS APCI: [M + H]+ m/z 285.3.
References:
WO2010/17046,2010,A1 Location in patent:Page/Page column 58